Microsurgical Treatment of a Large Through-and-Through Periapical Lesion with Apicomarginal Defect using Guided Tissue Regeneration (GTR): A Case Report of a Four-Year Follow-Up
October 15, 2024
J Endod Microsurg. 2024;3: 100015.
Under a Creative Commons license
HOW TO CITE THIS ARTICLE (AMA Referencing)
Popowicz W, Tkachenko O. Microsurgical treatment of a large through-and-through periapical lesion with apicomarginal defect using guided tissue regeneration (GTR): A case report of a four-year follow-up. J Endod Microsurg. 2024;3:100015. https://doi.org/10.23999/j.jem.2024.3.1
NATIONAL REPOSITORY OF ACADEMIC TEXTS
https://nrat.ukrintei.ua/en/searchdoc/2024U000229/
ABSTRACT. ABSTRACT IN UKRAINIAN (PDF). ABSTRACT IN POLISH (PDF).
In case of a long-term periapical lesion, destruction of both vestibular and oral cortical plates is sometimes observed and even a through-and-through periapical lesion occurs. The success of the treatment decreases when an apicomarginal defect is added to the through-and-through periapical lesion. Large periapical lesions should be treated initially by orthograde root canal therapy. When the signs and symptoms of the infection don’t recede after the treatment, then surgical approaches should be considered. In this case report, a 22-year-old female with previously initiated therapy was referred for an endodontic microsurgery of tooth 22 (i.e., upper left lateral incisor). After the endodontic treatment the patient was referred to the oral surgeon for apicoectomy with augmentation of the bone defect. The sinus tract in the apex area of the tooth 22 remained active since the surgical intervention. Endodontic microsurgery and guided tissue regeneration were performed. The article presents diagnostic data, namely pre- and post-operative images of cone beam computed tomography (after 2 and 4 years), as well as pre-, intra- and post-operative clinical images. All pre- and intraoperative procedures and stages are detailed. In particular, separation of platelet-rich fibrin (PRF) from venous blood, retrograde preparation with an ultrasonic tip and a device using a dental operating microscope and the use of a collagen membrane. After two- and four-year follow-up, radiographic examination revealed significant bone healing, and clinical signs and symptoms were absent. The patient hasn’t reported any symptoms since. The paper also analyzes scientific sources on the use of PRF and collagen membranes in bone defects of the jaws. Attention is also paid to the formation of a flap during operations of this type. The main six success factors in the treatment of such complex cases are highlighted. Rethinking the previously performed surgery (apicoectomy) in this patient, attention was paid to the main five factors that could contribute to the failure.
Keywords
Through-and-through periapical lesion, apicomarginal defect, sinus tract, endodontic microsurgery, guided tissue regeneration (GTR)
Edited by: Ievgen Fesenko, Kyiv Medical University, Ukraine. Reviewed by: Jignesh Rajguru, Ranjeet Deshmukh (RD) Dental College & Research Centre, India, Ievgen Fesenko, Kyiv Medical University, Ukraine, and Yan Vares, Danylo Halytsky Lviv National Medical University, Ukraine.
INTRODUCTION
The main cause of unsuccessful periapical healing after primary endodontic therapy or retreatment is the persistence of bacteria and infected tissue in the endodontic space [1]. The anterior region of the maxilla (especially lateral incisors) is the most common involved area [2]. In instances where nonsurgical retreatment cannot solve the problem a significant number of persistent nonhealing cases can be saved by endodontic microsurgery with a predictably favorable prognosis [3]. According to meta-analysis of the literature the success rate for traditional root-end surgery 59% and for endodontic microsurgery 94% respectively [4, 5]. By removing the diseased tissue, debriding the canal system, and sealing the defect or cavity, the surgeon prevents or reduces the spread of microorganisms within the periradicular tissues.
Regeneration of periapical defects may have a significant problem in periradicular surgery. In such circumstances, the gingival connective tissue can proliferate, or the oral epithelium can migrate into the defect, preventing the development of normal trabecular bone. Hard tissue can be restored using guided tissue regeneration (GTR) [6].
An apicomarginal defect is a mix of two communicating bone defects: a periapical bone defect plus a total root dehiscence [7]. These defects are associated with relatively lower success rates after endodontic surgery [8, 9]. It has been reported [10, 11] that, when the apex of the root is totally surrounded by bone, the success rate is higher than when there is a lack of one cortical bone plate (it decreases to 37%) [9] or two cortical bone plates (to 25%) [8].
Treatment of large periapical defects using GTR increases overall treatment success [12]. Use of GTR in endodontic surgery of through-and-through lesions that involve both the buccal and palatal alveolar cortical plates is recommended [13].
CASE REPORT
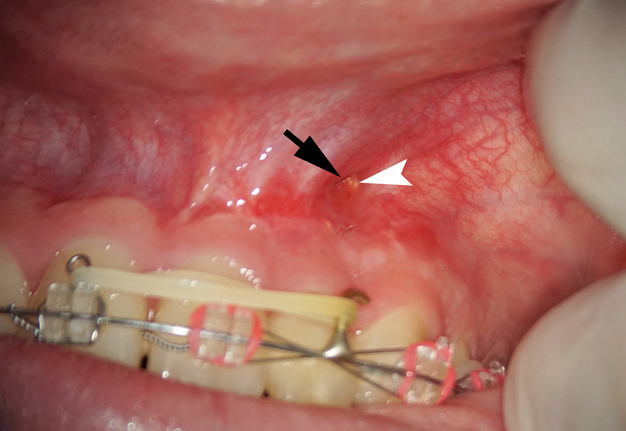
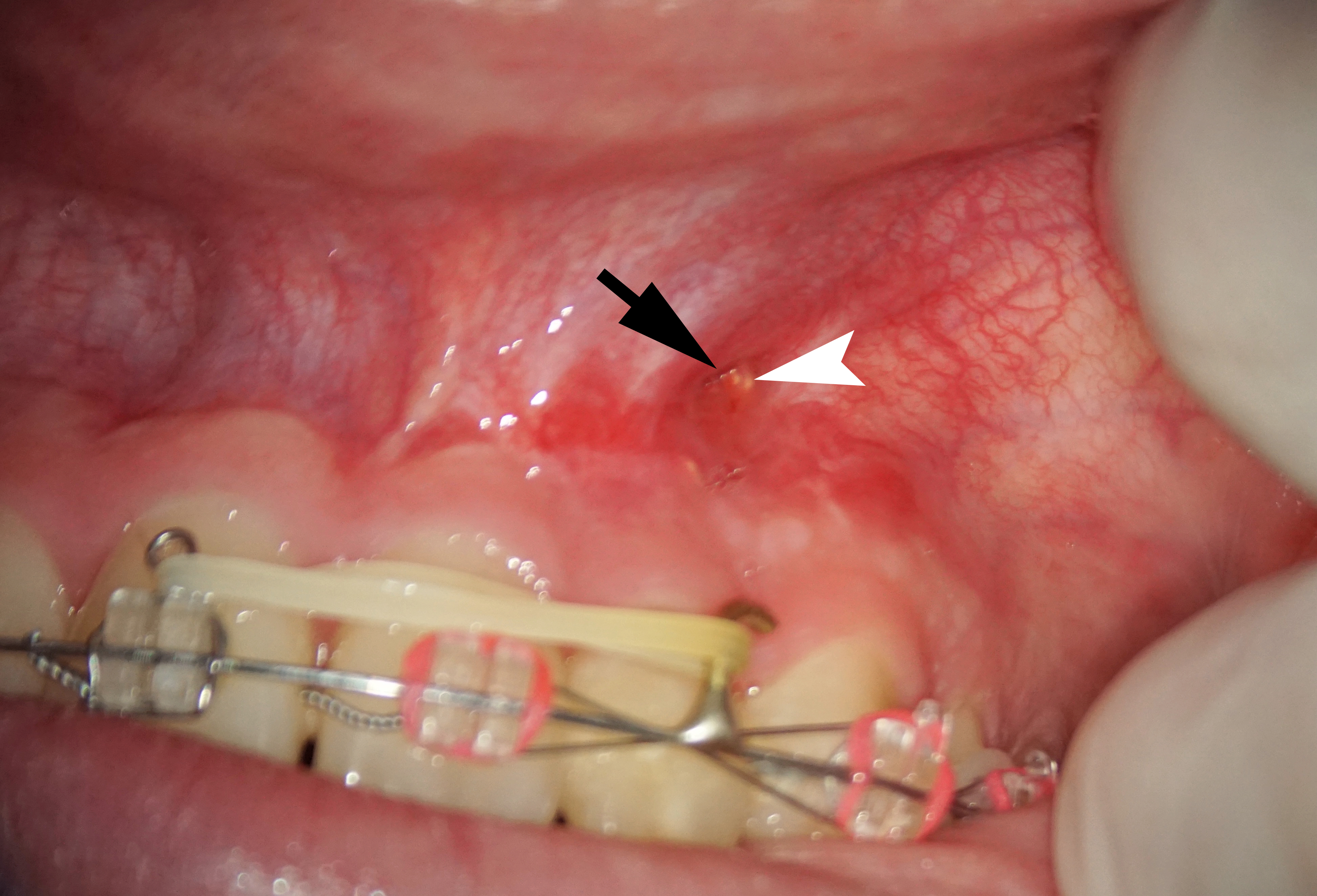
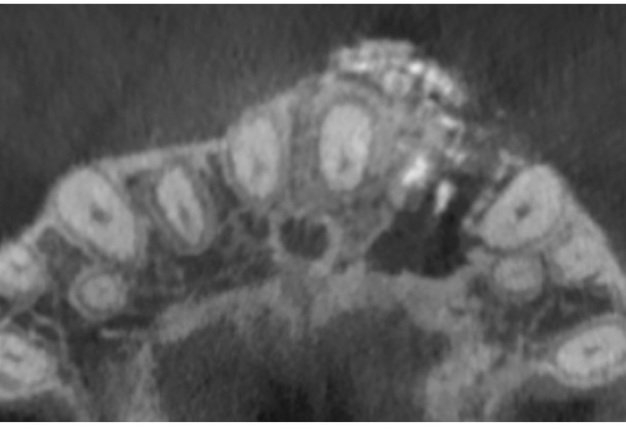
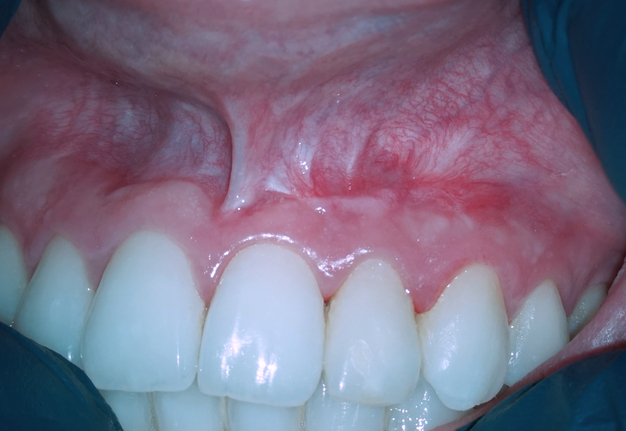
A 22-year-old female patient was referred for an endodontic microsurgery. Tooth 22 (i.e., upper left lateral incisor) was symptomatic, luxated (II degree). A sinus tract observed above the apex contained purulent exudation and xenograft debris (Fig 1). Periodontal probing depths around teeth 21, 22, 23 were within the normal range. The patient had had orthodontic treatment (fixed braces), but tooth 22 hadn’t been involved.
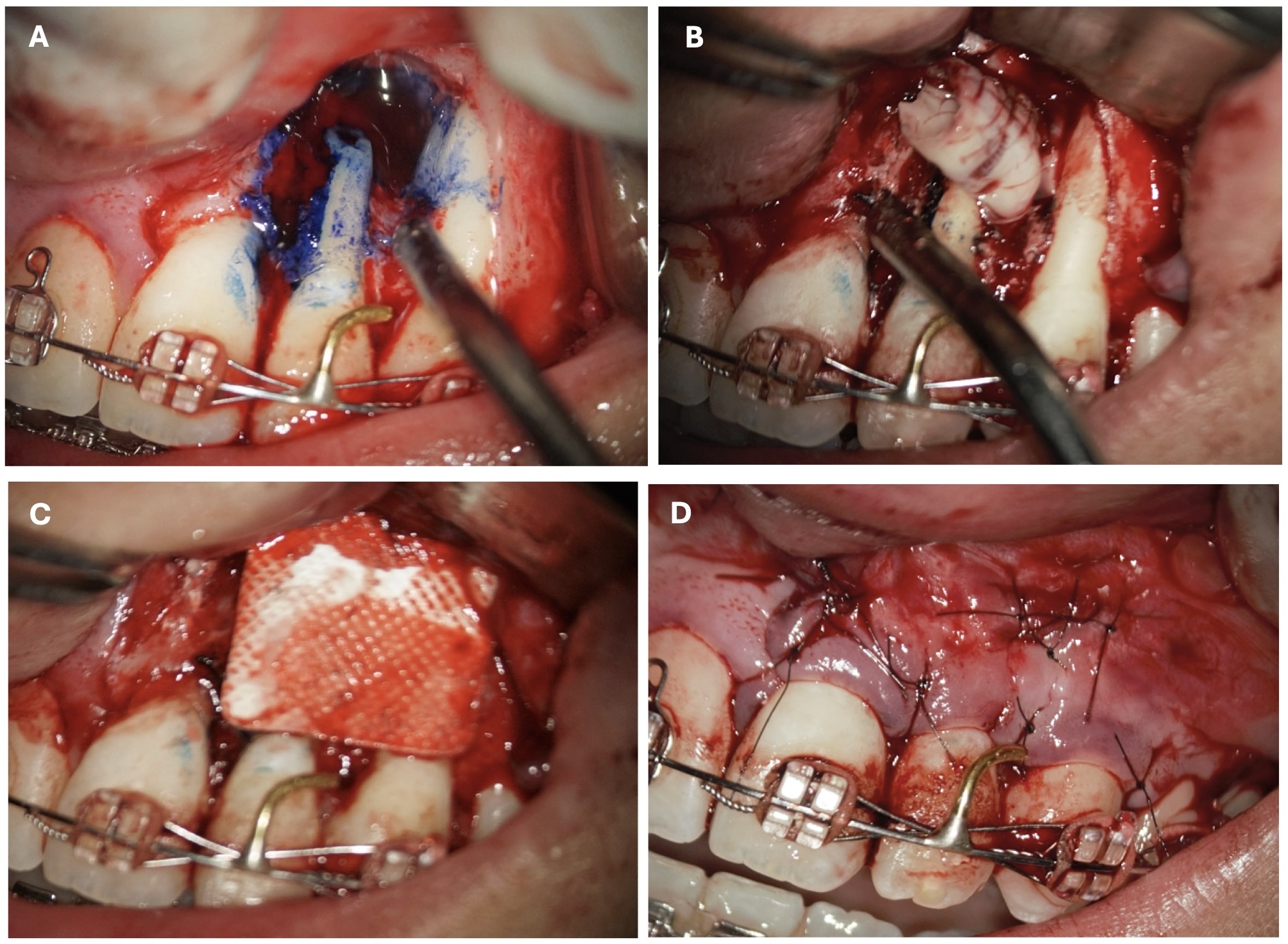
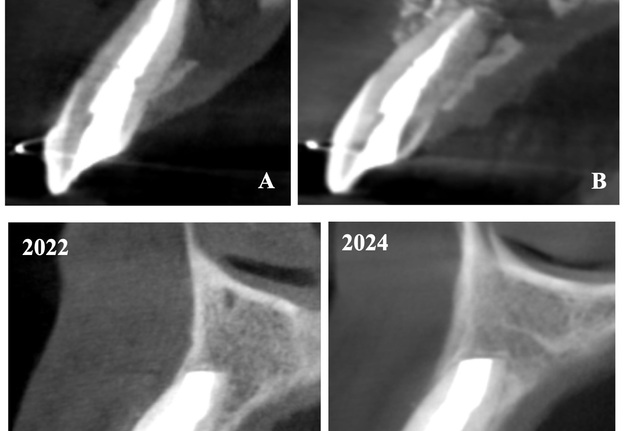
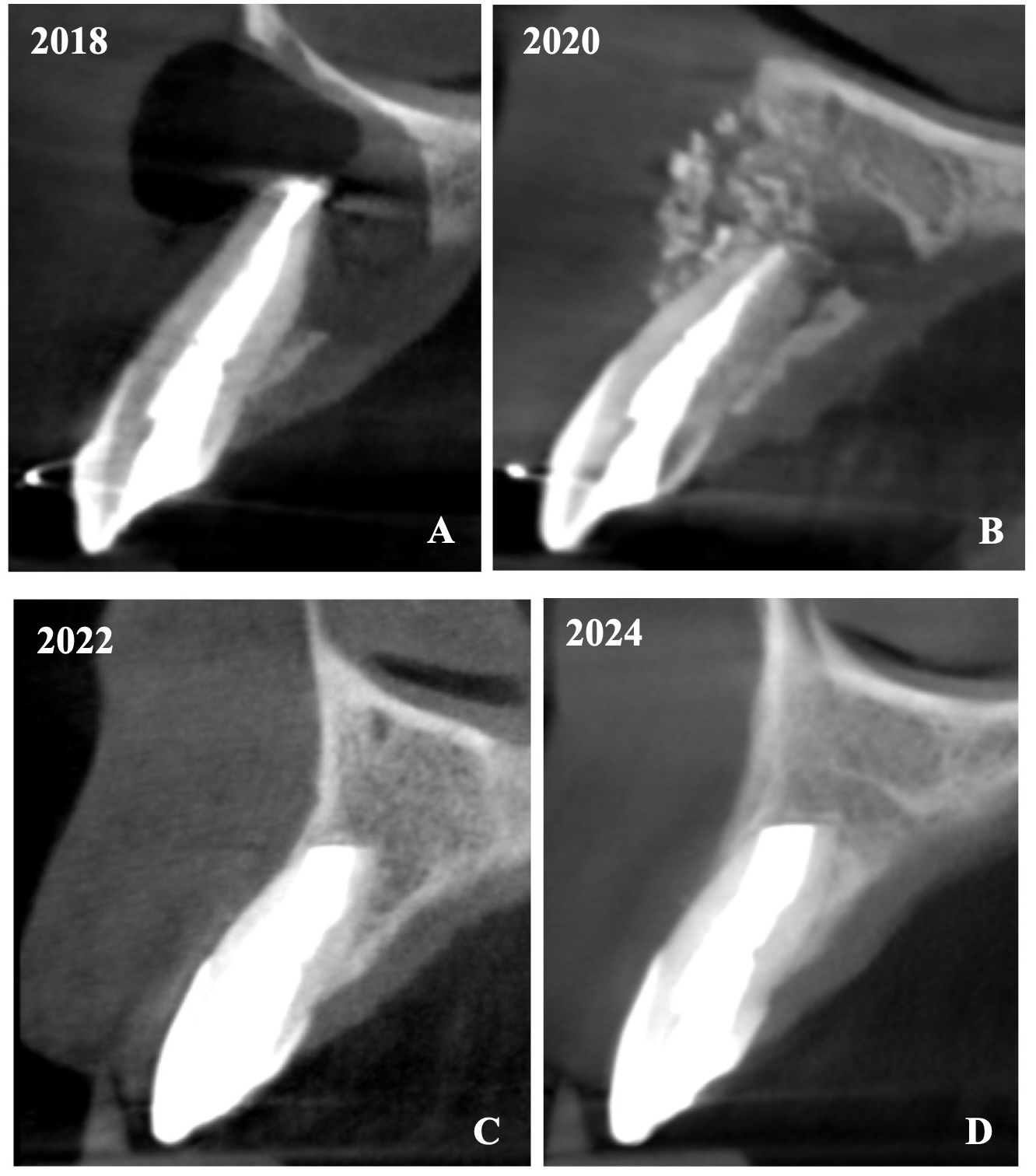
In anamnesis it was indicated that the patient had endodontic treatment of extensive lesion of tooth 22 on 23 August, 2018 (Fig 2). After it the patient was referred to the oral surgeon for apicoectomy with augmentation of the bone defect. The sinus tract in the apex area of tooth 22 remained active since the surgical intervention.
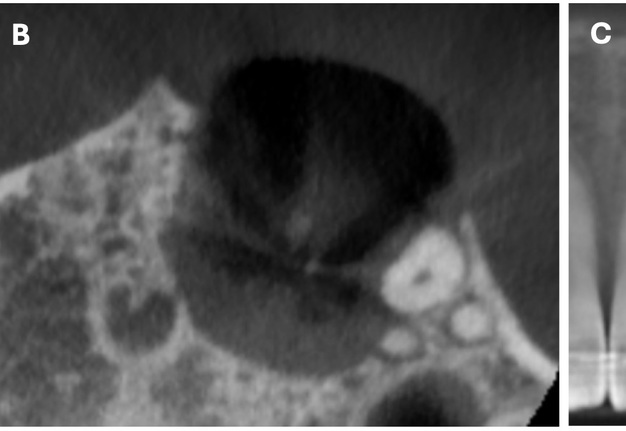
The cone beam computed tomography (CBCT) analysis as of 2020 revealed partial bone reconstruction in the palatal part of the defect in the apex area of tooth 21 (tooth is vital). The bone defect was filled with heterogeneous, contrasting material (xenograft) (Fig 3).
Preoperative Procedure
Before the surgical procedure, the patient’s venous blood (20 ml) was drawn via venipuncture of the antecubital vein. It was collected in four 10-ml sterile glass tubes coated with an anticoagulant (acidcitrate dextrose). The blood was centrifuged with Centurion PRO-PRP S (Centurion Scientific Limited, Chichester, West Sussex, UK) at the speed of 2700 rpm for 10 minutes to separate platelet-rich fibrin (PRF) from platelet-poor plasma. PRF was stored in a PRF box (Doctor Tools, Vladimirescu, Romania). A presurgical rinse with 0.2% solution of chlorhexidine (Eludril Classic; Pierre Fabre Group, Paris, France) was performed.
Surgical Procedure
The entire surgical procedure (W.P.) was performed using a dental operating microscope (Microscope Carl Zeiss EXTARO 300, Germany). Anesthesia was achieved with buccal infiltration of 3 capsules (5.4 ml) of 2% lidocaine hydrochloride with 1:50,000 epinephrine (Xylodont; Molteni Stomat, Florence, Italy). The full-thickness triangular flap was raised with vertical incision in frenulum and horizontal sulcular incision from tooth 21 to 24.
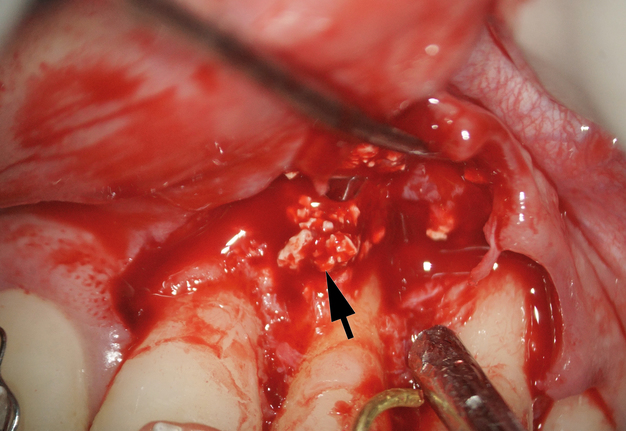
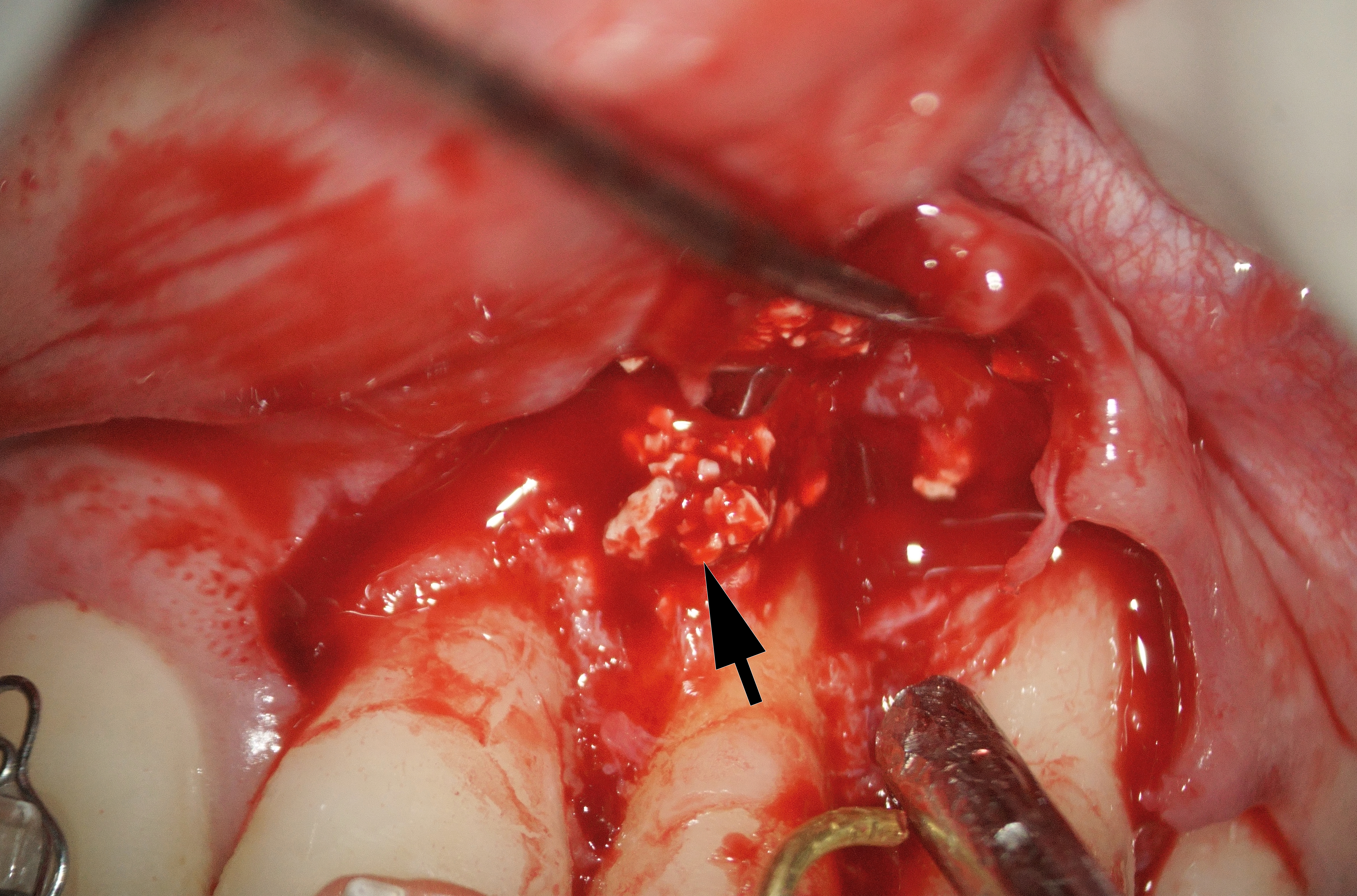
The bone defect was cleaned from a substantial amount of granulation soft tissue and loosed xenograft granules (Fig 4). An apicomarginal bone defect was detected (class 2B, purely endodontic origin, according to apicomarginal defects classification [14].
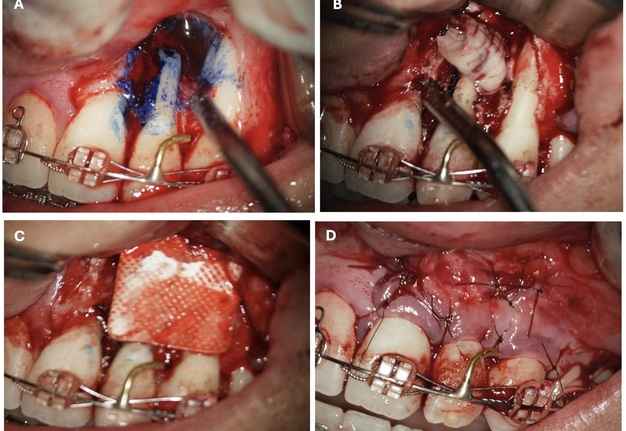
After cleaning the root section surface with a surgical bur (Lindemann H254E, Komet, Germany), the lack of retrofilling was identified. The vertical root fracture wasn’t identified with the help of dying with 1% aqueous solution of methylene blue Canal detector (Cerkamed, Poland) (Fig 5A). 3 mm-deep retrograde preparation with an ultrasonic tip and device was performed (E11D, Woodpecker, Guilin Zhuomuniao Medical Devices Co., China). The root canal was filled with MTA+ (Cerkamed, Poland).
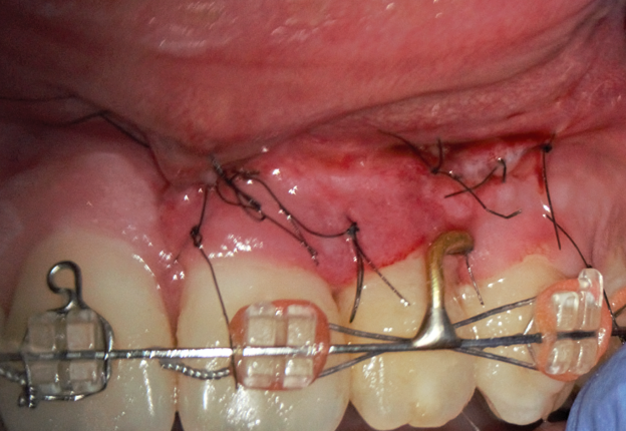
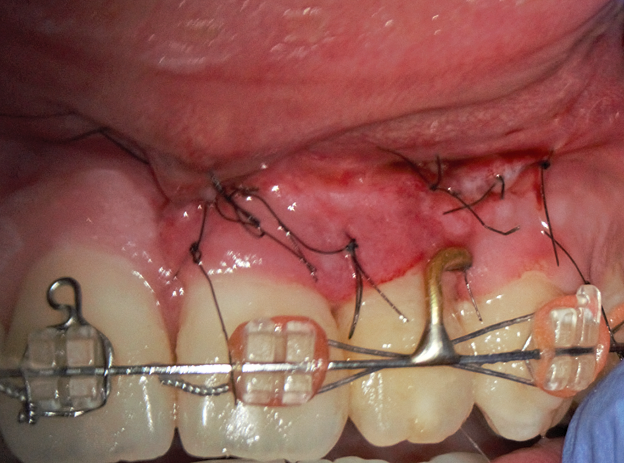
The bone defect was filled with a PRF plug (Fig 5B) and covered with a collagen membrane (SinossMem, B&B Dental Implant Company, Italy) (Fig 5C). It was covered with a PRF membrane and the wound was sutured with polypropylene (Luxylene 6/0, Lux-sutures S. A. Luxembourg) (Fig 5D).
FIGURE 5. The vertical root fracture wasn’t identified with the help of dying with 1% aqueous solution of methylene blue Canal detector (Cerkamed, Poland) (A). The bone defect was filled with a PRF plug (B) and covered with a collagen membrane (SinossMem, B&B Dental Implant Company, Italy) (C). It was covered with a PRF membrane and the wound was sutured with polypropylene (Luxylene 6/0, Lux-sutures S. A. Luxembourg) (D).
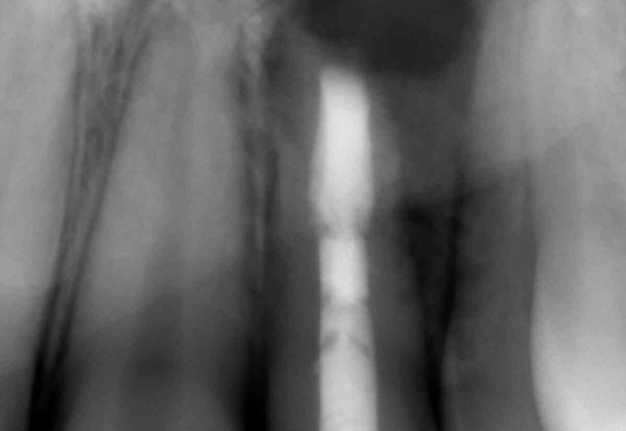
After the microsurgery X-ray was performed on 17 August, 2020 (Fig 6).
A follow-up which was carried out in 5 days revealed a sinus tract with serous exudation (Fig 7). The sutures were removed.
During subsequent visits gradual decrease of the sinus tract was observed. After 4 months the sinus tract closed completely (Fig 8).
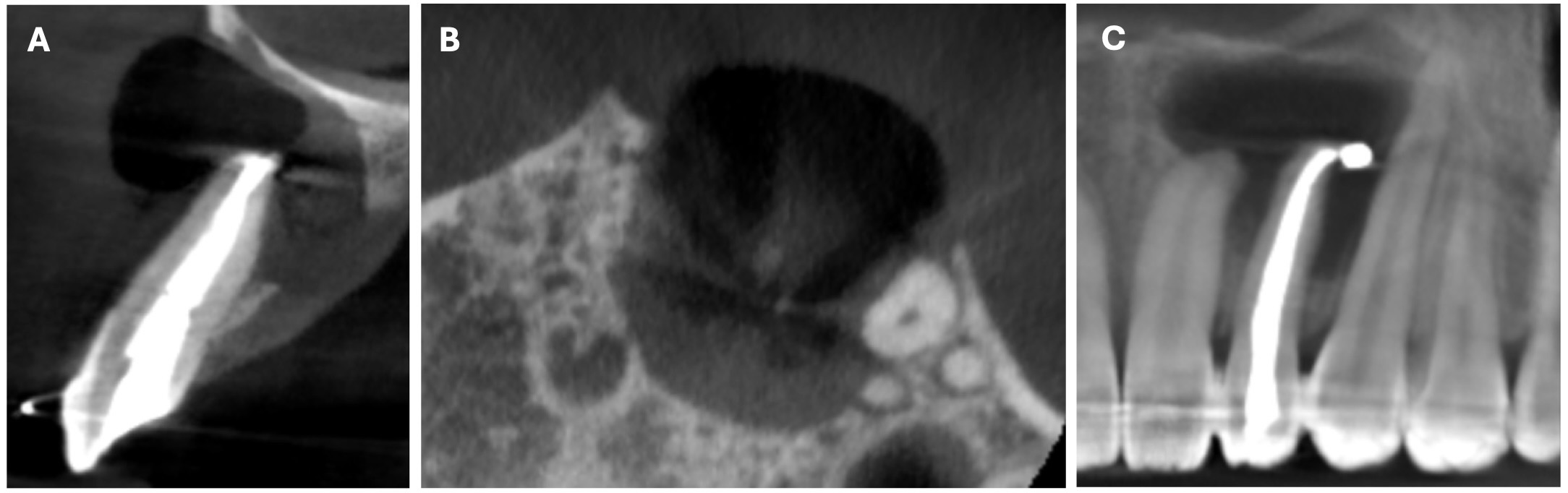
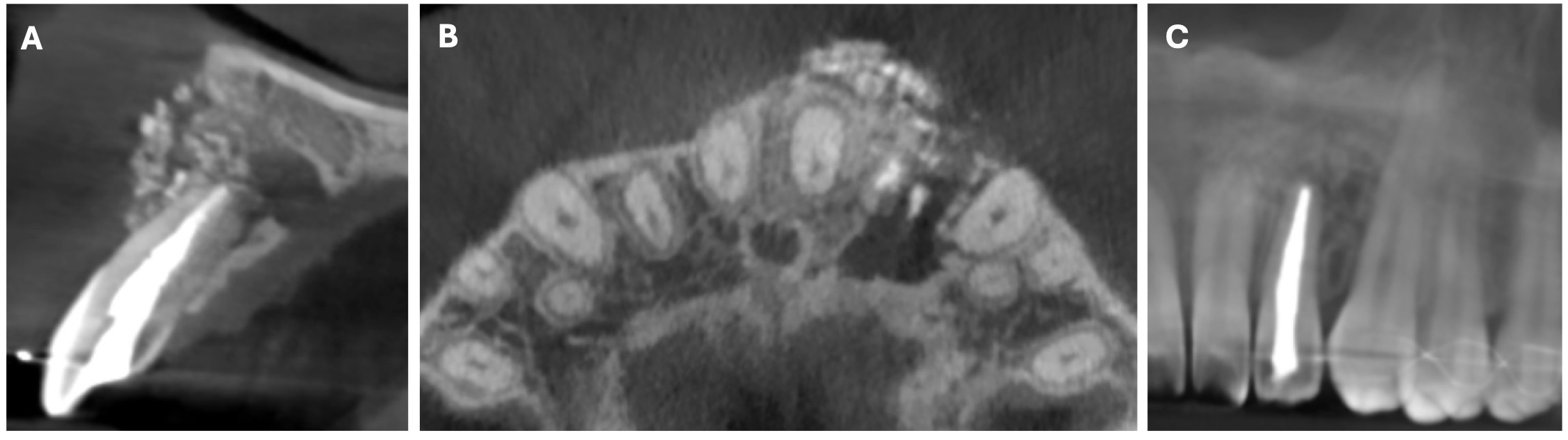
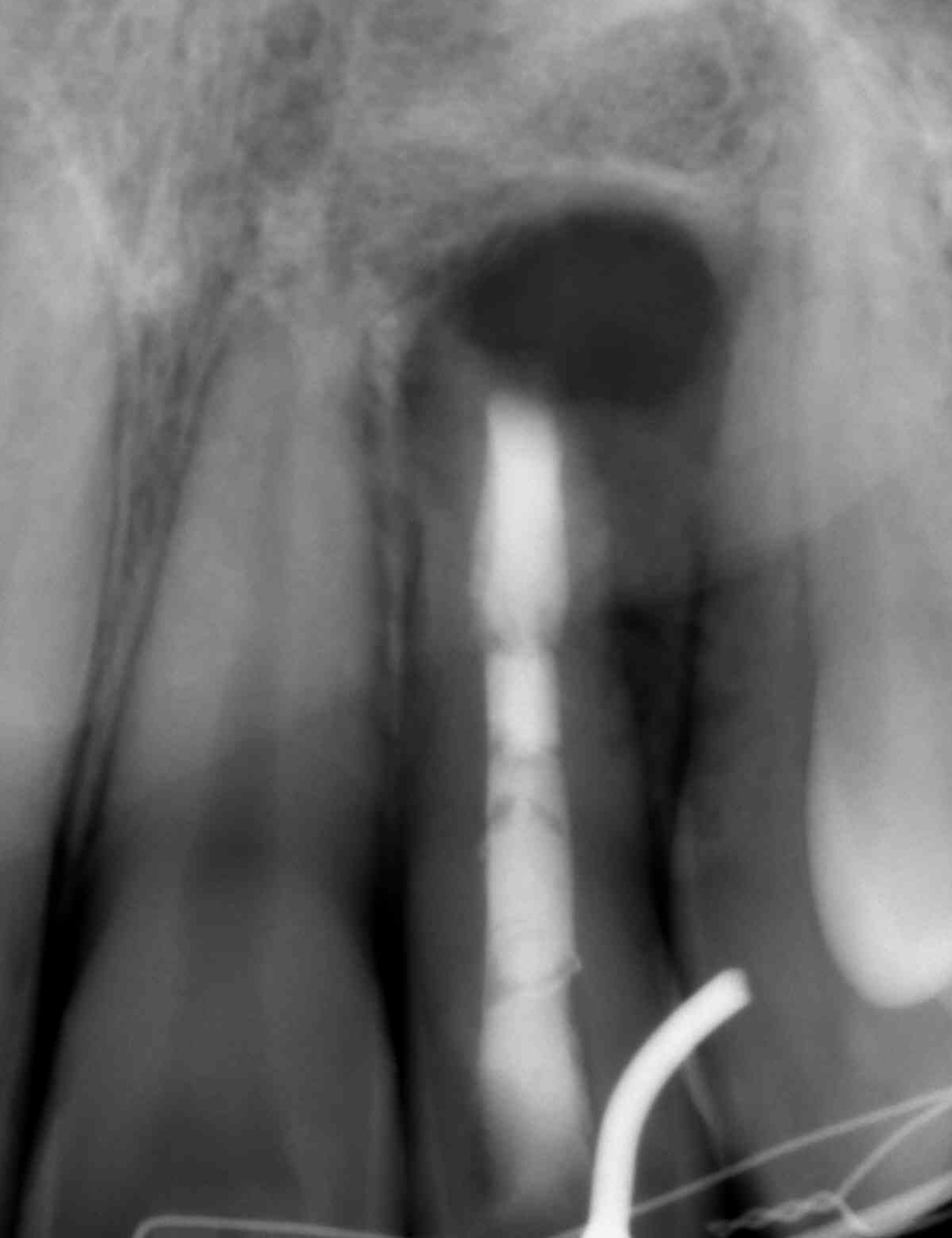
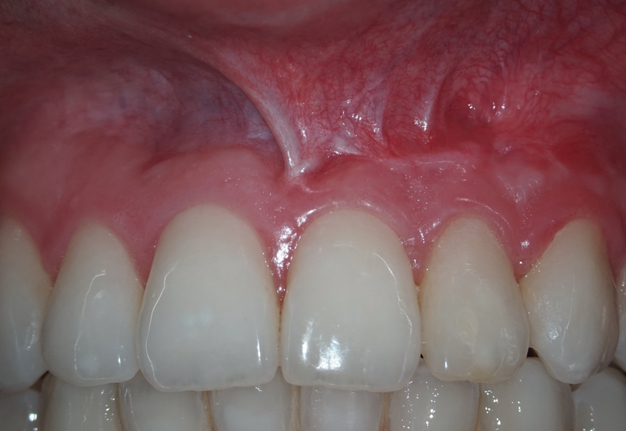
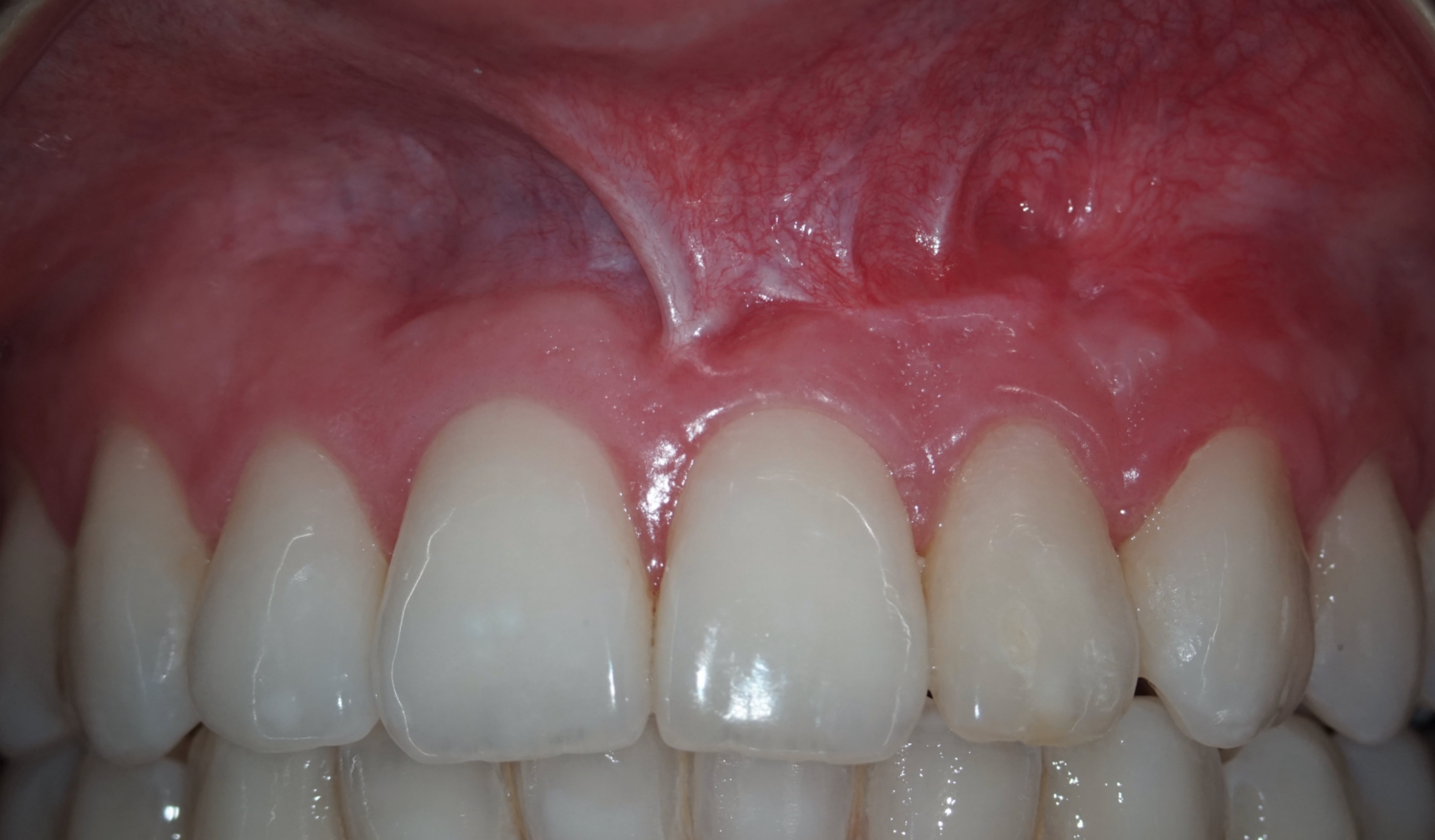
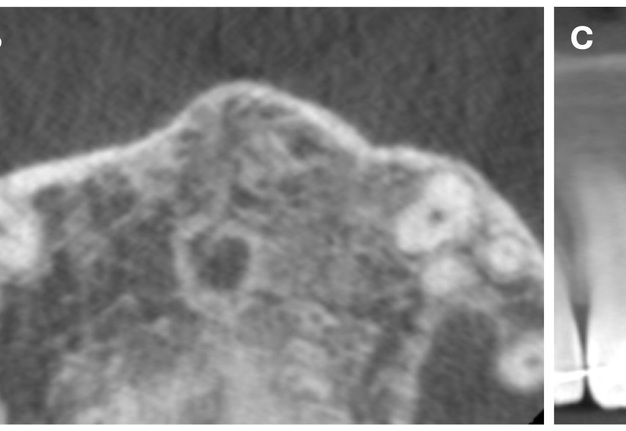
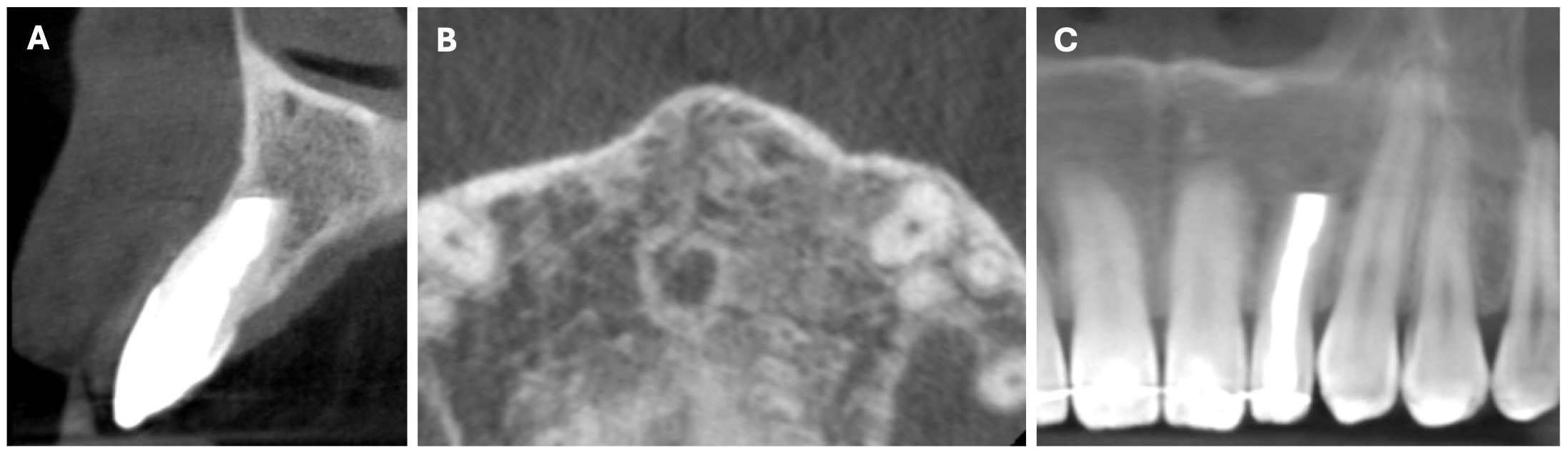
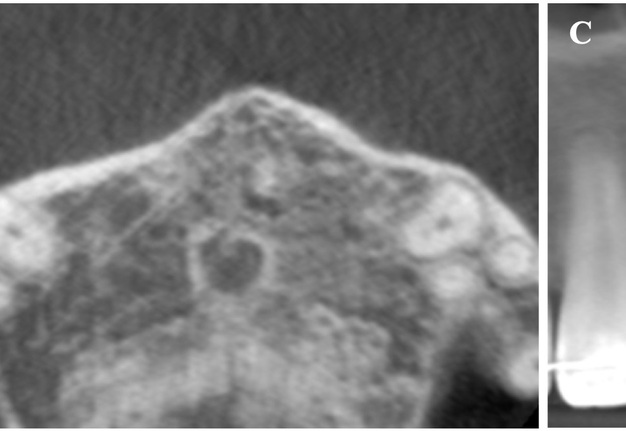
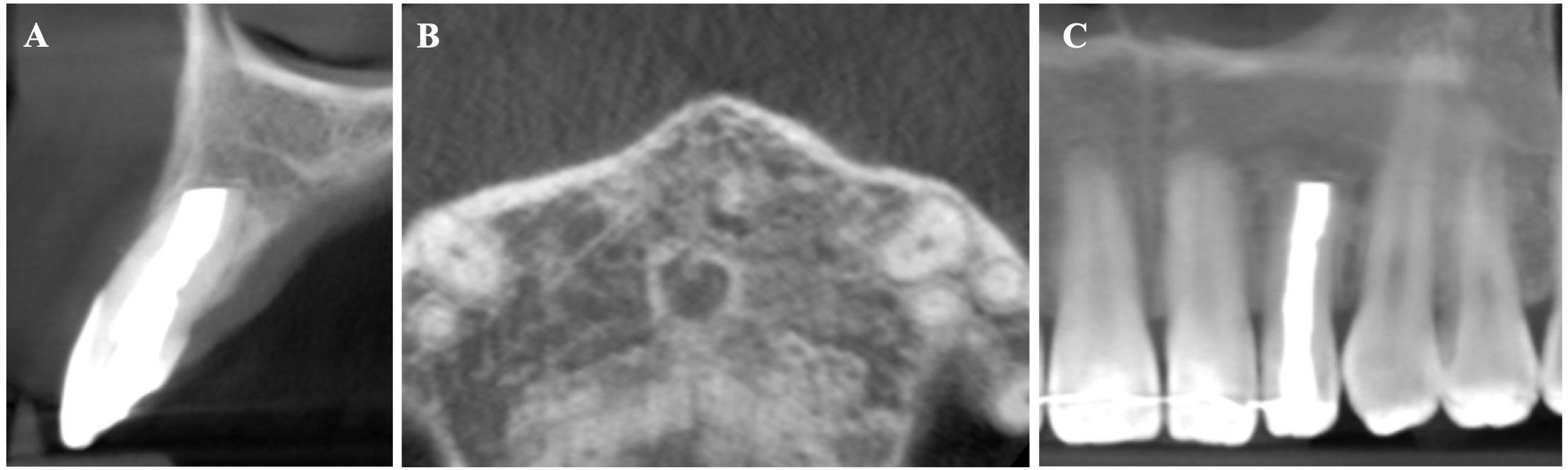
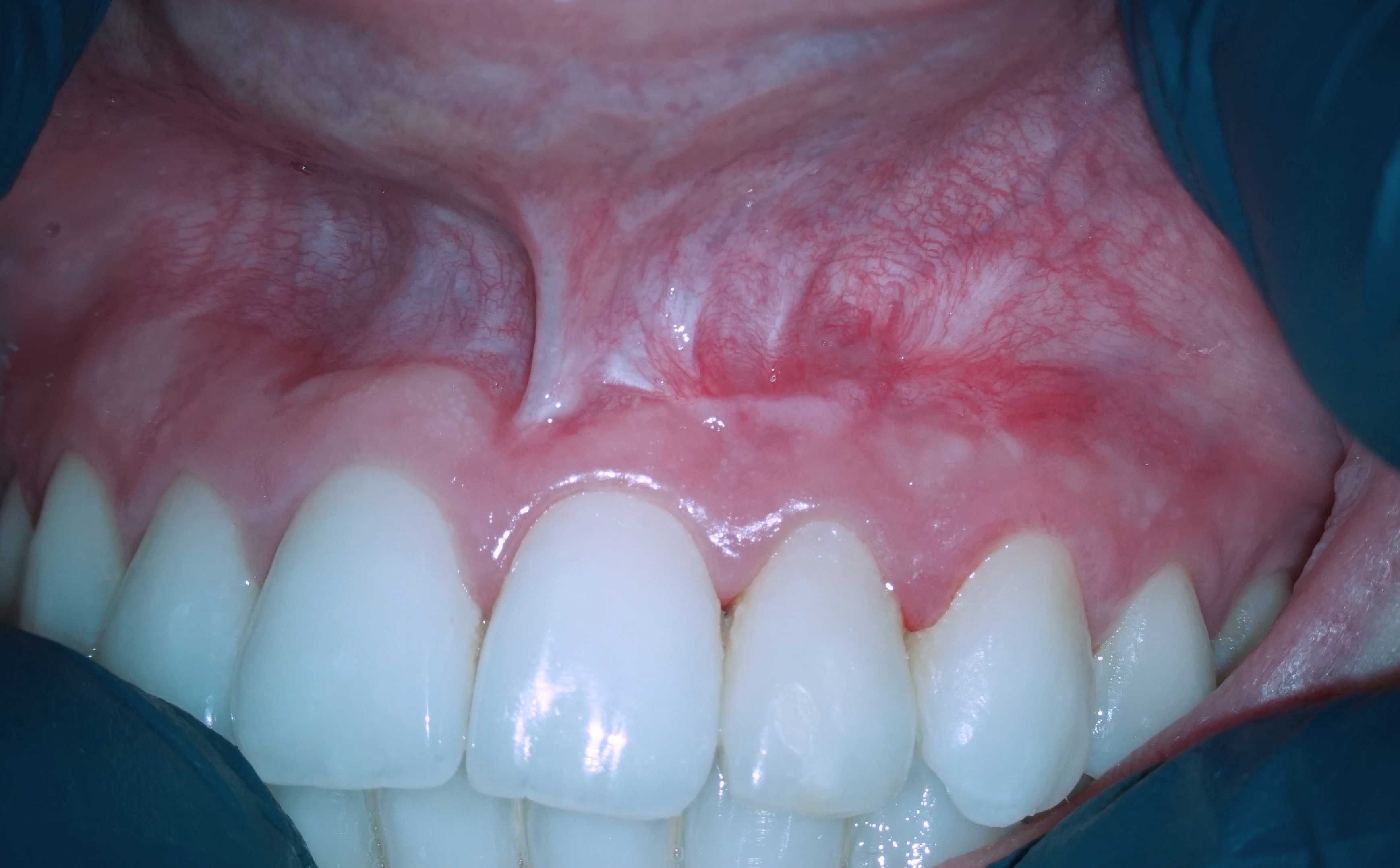
The CBCT made after 2 (i.e., in 2022) and 4 years (i.e., in 2024) revealed significant bone reconstruction (Figs 9 and 10). The patient hasn’t reported any symptoms since. Intraoral view four years (i.e., in 2024) after the endodontic microsurgery shows no signs of inflammation or sinus tract (Fig 11).
DISCUSSION
This case perfectly shows that a success in treatment of such complex cases depends on many factors. The main ones are:
-
Understanding the cause-and-effect relationships in the development of inflammation and why failure occurred after the first surgical treatment.
-
Analysis of evidence-based articles and guidelines that provide recommendations on how to conduct treatment according to the patient's problem.
-
Technical provision of all necessary tools, materials and equipment for endodontic microsurgery.
-
Clinical experience and good manual skills of a surgeon.
-
Use of materials and methods of treatment that have the highest success rate according to the scientific evidence-based literature.
-
Factors that depend on a patients themselves (health condition, complexity of a clinical case, their responsibility, a desire to save a tooth).
Analyzing the first performed surgical manipulation (apicoectomy), we should pay attention to the main factors that contribute to the failure:
-
Insufficient cleaning and tightness of the filling mass in the root canal. The most common cause of failure in nonsurgical endodontic treatment is a leaky canal (30.4%) [15].
-
Resection of the root tip was performed without retrograde preparation and filling. Song et al. [16] determined that no root-end filling and incorrect root-end preparation were the most common causes of failure, followed by missing or leaky canals and unidentified isthmuses.
-
Absence of membranes in case of a through-and-through bone defect. Application of barrier membranes in through-and-through bony crypts after endodontic surgery might create a microenvironment, which is conducive for osteogenesis in a short-term experimental observation or clinical follow-up as compared to without barrier membranes [17]. Based on limited information in the literature, through-and-through bone defects could benefit from application of GTR technique using bioabsorbable barrier membranes after endodontic surgery to improve the rate of new bone formation in short-term observation [18–20].
-
Improper use of bone-plastic material that sometimes masked chronic inflammation for a long time. Radiographically, the problem of using bone graft substitutes in endodontic surgery is the difficulty of differentiating incomplete healing (scar tissue) from uncertain healing (no healing) because bone graft substitutes are radiopaque [21].
-
Absence of control observations of clinical symptoms after the operation, the condition of the sinus tract, and the lack of control X-rays by a doctor who previously performed apicoectomy.
In periapical surgery the sulcular full thickness flap is often used [22]. The main disadvantage of the sulcular full thickness flap is recession and, especially, unpredictable shrinkage of the papilla during healing [23]. The risks of these complications are greater, especially when surrounding bone tissue is lost.
When discussing the issue of the rationality of filling a bone defect, it is worth noting the scientific data related to the use of PRF. Platelet-rich plasma (PRP), bone morphogenic proteins (BMPs), platelet-derived growth factor (PDGF), parathyroid hormone (PTH), and enamel matrix proteins (EMD) have been locally applied to promote the healing potential of the surgical site [24]. It has been advocated that PRF can be considered a healing biomaterial because it is constituted by a fibrin network in which platelets, leukocytes, cytokines and stem cells are enmeshed [25]. Moreover, the platelets in the PRF network are capable of slowly releasing PDGF and insulin-like growth factor (IGF), [26, 27] even up to one week [28]. The osteogenic potential of these molecules has been already demonstrated [29, 30]. PRF can be thought as a grow factor reservoir that can be employed without exposing the patient to any immunogenicity or infection risk [31].
A collagen graft can be another alternative; however, PRF has been proven to have a beneficial effect in regeneration [32–34].
The article [35] analyzes the use of PRF in endodontic microsurgery. A control group of four patients (without PRF) and a test group of seven patients (with PRF) were involved. After endodontic microsurgery, the results of both groups were compared. Then the assessment was carried out according to three important indicators: the speed of healing, the intensity of pain and the amount of swelling. In the group where PRF was used, a statistically significant differences in the three criteria were observed: the speed of periapical healing accelerated, the intensity of postoperative pain and the severity of postoperative swelling decreased.
Sometimes scar tissue formation with through-and-through periapical lesions during tissue repair is observed [36–38].
Ingrowth of connective tissue into the osseous defect prevents periapical bone regeneration. It can result in periapical scarring, which is often misdiagnosed as pathology and may lead to unnecessary surgical reentry by a practitioner who is not fully aware of the history. When the barrier membranes are placed over bony defects and closely adapted to the surrounding bone surface, an environment that prevents invasion of competing nonosteogenic cells from the overlying soft tissues can be created. This environment provides the bony defect time to heal [39]. The use of GTR principles [40] enhanced the quality and quantity of bone regeneration in large periapical defects, especially in through-and-through lesions [41].
Summing up this article, we would like to show in Figure 12 a comparison of sagittal CBCT scans of tooth 22 with different treatment by different doctors with an assessment of long-term results two and four years after microsurgical treatment.
CONCLUSION
The presented case report describes a difficult case that was resolved by endodontic microsurgery a positive outcome of which was enhanced by a two- and four-year follow-up. The use of PRF as an autologous graft in combination with a collagen membrane ensured complete healing, a good aesthetic result of soft tissues and the absence of any clinical signs and symptoms. Future long-term clinical observations and studies are needed to prove the effectiveness, predictability and success of this technique.
CONFLICT OF INTEREST
The authors declare that they don’t have any conflicts of interest.
Open Researcher and Contributor ID (ORCID)
Witold Popowicz: https://orcid.org/0009-0002-1499-3798
Oleksandr Tkachenko: https://orcid.org/0000-0003-2582-4551
REFERENCES (41)
-
Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15(6):348-381. https://doi.org/10.1177/154411130401500604
-
Koseoglu BG, Atalay B, Erdem MA. Odontogenic cysts: a clinical study of 90 cases. J Oral Sci. 2004;46(4):253-257. https://doi.org/10.2334/josnusd.46.253
-
Kim S, Kratchman S, Guess G. Endodontics: Colleagues for excellence newsletter. Contemporary endodontic microsurgery. American association of endodontists; Fall 2010.
-
Setzer FC, Shah SB, Kohli MR, et al. Outcome of endodontic surgery: a meta-analysis of the literature–part 1: comparison of traditional root-end surgery and endodontic microsurgery. J Endod. 2010;36(11):1757-1765. https://doi.org/10.1016/j.joen.2010.08.007
-
Nozhenko OA. Review of “Outcome of endodontic surgery: a meta-analysis of the literature—part 1: comparison of traditional root-end surgery and endodontic microsurgery” by Setzer and colleagues in J Endod 36(11):1757-1765, 2010. J Endod Microsurg. 2023;2:41-42. https://doi.org/10.23999/jem.2023.2.5
-
Alajmi В, Karobari М, Aldowah О. Treatment of a large through and through periapical lesion using guided tissue regeneration: A case report of 2 years follow-up. Clin Case Rep. 2022;10(10):e6405. https://doi.org/10.1002/ccr3.6405
-
Merino E. Endodontic microsurgery. 1st ed. New Malden: Quintessence Pub; 2009.
-
Hirsch JM, Ahlstrom U, Henrikson PA, et al. Periapical surgery. Int J Oral Surg. 1979; 8(3):173-185. https://doi.org/10.1016/s0300-9785(79)80016-2
-
Skoglund A, Persson G. A follow-up study of apicoectomized teeth with total loss of the buccal bone plate. Oral Surg Oral Med Oral Pathol. 1985;59(1):78-81. https://doi.org/10.1016/0030-4220(85)90120-3
-
Rud J, Andreasen JO. A study of failures after endodontic surgery by radiographic, histologic and stereomicroscopic methods. Int J Oral Surg. 1972;1(6):311-328. https://doi.org/10.1016/s0300-9785(72)80052-8
-
Molven O, Haise A, Grung B. Surgical management of endodontic failures: indications and treatment results. Int Dental J. 1991;41(1):33-42.
-
Tsesis I, Rosen E, Tamse A, et al. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment: a systematic review and meta-analysis. J Endod. 2011;37(8):1039-1045. https://doi.org/10.1016/j.joen.2011.05.016
-
Pecora G, Baek SH, Rethnam S, Kim S. Barrier membrane techniques in endodontic microsurgery. Dent Clin N Am. 1997;41(3):585-602.
-
Dietrich T, Zunker P, Dietrich D, Bernimoulin JP. Apicomarginal defects in periradicular surgery: classification and diagnostic aspects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(2):233-239. https://doi.org/10.1067/moe.2002.123864
-
Song M. Kim HC, Lee W, Kim E. Analysis of the cause of failure in nonsurgical endodontic treatment by microscopic inspection during endodontic microsurgery. J Endod. 2011;37(11):1516-1519. https://doi.org/10.1016/j.joen.2011.06.032
-
Song M, Jung IY, Lee SJ, et al. Prognostic factors for clinical o comes in endodontic microsurgery: A retrospective study. J Endod. 2011;37(7):927-933. https://doi.org/10.1016/j.joen.2011.04.005
-
Tsesis I. Complications in endodontic surgery. Prevention, identification and management. 1st ed. Berlin Heidelberg: Springer-Verlag; 2014.
-
Tsesis I, Rosen E, Tamse A, et al. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment: a systematic review and metaanalysis. J Endod. 2011;37(8):1039-1045. https://doi.org/10.1016/j.joen.2011.05.016
-
von Arx T, Cochran DL. Rationale for the application of the GTR principle using a barrier membrane in endodontic surgery: a proposal of classification and literature review. Int J Periodontics Restorative Dent. 2001;21(2):127-139.
-
von Arx T, AlSaeed M. The use of regenerative techniques in apical surgery: A literature review. Saudi Dent J. 2011;23(3):113-127. https://doi.org/10.1016/j.sdentj.2011.02.004
-
Taschieri S, del Fabbro M, Testori T, et al. Efficacy of guided tissue regeneration in the management of through-and-through lesions following surgical endodontics: a preliminary study. Int J Periodontics Restorative Dent. 2008;28(3):265-271.
-
Beer R, Baumann MA, Kim S. Endodontology. Stuttgart: Thieme Verlag; 2000.
-
Velvart P, Ebner-Zimmermann U, Pierre Ebner J. Papilla healing following sulcular full thickness flap in endodontic surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(3):365-369. https://doi.org/10.1016/s1079210404002598
-
Bashutski JD, Wang HL. Periodontal and endodontic regeneration. J Endod. 2009;35(3):321-328.https://doi.org/10.1016/j.joen.2008.11.023
-
Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate, Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299-303. https://doi.org/10.1016/j.tripleo.2005.07.012
-
Clausen C, Hermund NU, Donatsky O, et al. Homologous activated platelets stimulate differentiation and proliferation of primary human bone cells. Cells Tissues Organs. 2006;184(2):68-75. https://doi.org/10.1159/000098948
-
Ferreira CF, Carriel Gomes MC, Filho JS, et al. Platelet-rich plasma influence on human osteoblasts growth. Clin Oral Implants Res. 2005;16(4):456-460. https://doi.org/10.1111/j.1600-0501.2005.01145.x
-
Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63-69. https://doi.org/10.1080/08977190802636713
-
Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. Chir Organi Mov. 2008;92(3):161-168. https://doi.org/10.1007/s12306-008-0064-1
-
Kawai M, Rosen CJ. Insulin-like growth factor-I and bone: Lessons from mice and men. Pediatr Nephrol. 2009;24(7):1277-1285. https://doi.org/10.1007/s00467-008-1040-6
-
Jang ES, Park JW, Kweon H, et al. Restoration of peri-implant defects in immediate implant installations by Choukroun platelet-rich fibrin and silk fibroin powder combination graft. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(6):831-836.https://doi.org/10.1016/j.tripleo.2009.10.038
-
Vares Y, Binderman I, Galyant K. Traumatic mandibular cyst defect grafted with autologous dentin and platelet-rich fibrin composite: a case report. Int J Periodontics Restorative Dent. 2022;42(2):253-259. https://doi.org/10.11607/prd.5215
-
Popowicz W, Palatynska-Ulatowska A, Kohli M.R. Targeted endodontic microsurgery: computed tomography–based guided stent approach with platelet-rich fibrin graft: A report of 2 cases. J Endod. 2019;45(12):1535-1542. https://doi.org/10.1016/j.joen.2019.08.012
-
Canellas JV, Medeiros PJ, Figueredo CM, et al. Platelet-rich fibrin in oral surgical procedures: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2018;48(3):395-414. https://doi.org/10.1016/j.ijom.2018.07.007
-
Angerame D, Biasi M, Kastrioti I, et al. Application of platelet-rich fibrin in endodontic surgery: a pilot study. G Ital Endod. 2015;29(2):51-57. https://doi.org/10.1016/j.gien.2015.08.003
-
Andreasen JO, Rud J. Modes of healing histologically after endodontic surgery in 70 cases. Int J Oral Surg. 1972;1(3):148-160. https://doi.org/10.1016/s0300-9785(72)80005-x
-
Rud J, Andreasen JO, Jensen JF. A multivariate analysis of the influence of various factors upon healing after endodontic surgery. Int J Oral Surg. 1972;1(5):258-271.https://doi.org/10.1016/s0300-9785(72)80045-0
-
Tkachenko O, Volokitin A. A clinical case of endodontic microsurgery with a histological diagnosis of an apical scar. J Endod Microsurg. 2023;2:2-23. https://doi.org/10.23999/jem.2023.2.2
-
Pecora G, Baek S, Rethnam S, Kim S. Barrier membrane techniques in endodontic microsurgery. Dent Clin North Am.1997;41(3):585-602.
- Sorokivskyi IS. Elimination of acute post-extraction oroantral communications using modern biomaterials for guided tissue regeneration. Dissertation. Danylo Halytsky Lviv National Medical University; 2018. In Ukrainian.
-
Pecora G, Kim S, Celletti R, Davarpanah M. The guided tissue regeneration principle in endodontic surgery: One-year postoperative results of large periapical lesions. Int Endod J. 1995;28(1):41-46. https://doi.org/10.1111/j.1365-2591.1995.tb00155.x
ABSTRACT IN UKRAINIAN (PDF). АНОТАЦІЯ
При тривалому періапікальному ураженні іноді спостерігається деструкція як вестибулярної, так і оральної кортикальних пластинок і навіть наскрізне периапікальне ураження. Успіх лікування знижується, коли до наскрізного периапікального ураження додається апікомаргінальний дефект. Великі периапікальні ураження слід спочатку лікувати за допомогою ортоградної терапії кореневих каналів. Якщо ознаки та симптоми інфекції не зникають після лікування, слід розглянути можливість хірургічного втручання. У цьому випадку 22-річна жінка з раніше розпочатим лікуванням була направлена на ендодонтичну мікрохірургію зуба 22 (тобто верхнього лівого бокового різця). Після ендодонтичного лікування пацієнтку направили до орального хірурга для апікоектомії та заміщення кісткового дефекту. Синус тракт (тобто нориця) в ділянці верхівки зуба 22 з моменту хірургічного втручання залишився активним. Виконано ендодонтичну мікрохірургію та керовану тканинну регенерацію. В статті представлено дані діагностики, а саме перед- та післяопераційні зображення конусно-променевої комп’ютерної томографії (через 2 та 4 роки), а також перед-, ітра- та післяопераційні клінічні зображення. Деталізовано всі перед- та інтраопераційні процедури і етапи. Зокрема, відокремлення з венозної крові збагаченого тромбоцитами фібрину (PRF, акронім англомовного терміну “platelet-rich fibrin”), ретроградне препарування з ультразвуковим наконечником і пристроєм із застосуванням стоматологічного операційного мікроскопу та використання колагенової мембрани для керованої тканинної регенерації (КТР) (синонім: направлена тканинна регенерація). Після дво- та чотирирічного спостереження рентгенологічне дослідження виявило загоєння, а клінічні ознаки та симптоми були відсутні. Відтоді пацієнт не повідомляв про жодні симптоми. В статті також аналізуються наукові джерела із застосування збагаченого тромбоцитами фібрину при кісткових дефектах щелеп та колагенових мембран. Також приділено увагу до формування клаптя при операціях такого типу. Виділено основні 6 фактори успіху в лікуванні таких складних випадків. Переосмислюючи попередньо виконану хірургічну маніпуляцію (апікоектомію) у даної пацієнтки, звернено увагу на основні 5 факторів, що могли сприяти невдачі.
КЛЮЧОВІ СЛОВА
Наскрізне періапікальне ураження, апікомаргінальний дефект, синус тракт, ендодонтична мікрохірургія, керована тканинна регенерація (КТР)
ABSTRACT IN POLISH (PDF). STRESZCZENIE
Długotrwałe zapalne zmiany okołowierzchołkowe mogą prowadzić do uszkodzenia blaszki zbitej po stronie przedsionkowej jak i podniebiennej, a nawet do przezwyrostkowej perforacji wyrostka. Współistnienie zmiany okołowierzchołkowej perforującej wyrostek z całkowitą pionową utratą blaszki pogarsza rokowanie leczenia. W przypadku rozległych zmian okołowierzchołkowych leczeniem z wyboru jest leczenie kanałowe. Dopiero w przypadku utrzymywania się objawów radiologicznych i/lub klinicznych stanu zapalnego, należy rozważyć leczenie chirurgiczne. W artykule zaprezentowano przypadek 22-letniej pacjentki u której wykonano najpierw standardowe leczenie kanałowe zęba 22 a następnie została skierowana do chirurga na zabieg resekcji wierzchołka i odbudowę ubytku kostnego materiałem ksenogennym. Po przeprowadzonym leczeniu chirurgicznym z uwagi na utrzymującą się aktywną przetokę ropną w okolicy wierzchołka zęba 22 pacjentkę skierowano na zabieg mikrochirurgii endodontycznej z zastosowaniem sterowanej regeneracji tkanek (z wypełnieniem ubytku kostnego osoczem bogatopłytkowym). W artykule zaprezentowano przebieg leczenia, począwszy od procedur diagnostycznych, poprzez pełną procedurę zabiegu mikrochirurgii endodontycznej oraz wyniki czteroletniej obserwacji. Przedstawiono przed- i pozabiegowe (po 2 i 4 latach) obrazy z CBCT a także przed-, śród- i pozabiegowe zdjęcia kliniczne. Szczegółowo opisano wszystkie procedury i etapy przed- i śródzabiegowe, w szczególności przygotowanie fibryny bogatopłytkowej (PRF) z krwi żylnej, preparację wsteczną za pomocą końcówki ultradźwiękowej z użyciem mikroskopu zabiegowego oraz zastosowanie błony zaporowej. Czteroletnia obserwacja kliniczna i radiologiczna wykazała ustąpienie objawów, wygojenie przetoki ropnej oraz odbudowę kości wyrostka zębodołowego szczęki. W artykule przeprowadzona została analiza naukowa stosowania PRF i błon kolagenowych w ubytkach kostnych szczęki i żuchwy. Zwrócono również uwagę na technikę preparacji płata podczas tego typu zabiegów. Wyróżniono sześć głównych czynników decydujących o powodzeniu w leczeniu tak złożonych przypadków. Analizując uprzednio wykonany zabieg chirurgiczny apicektomii u pacjentki, zwrócono uwagę na pięć głównych czynników, które mogły przyczynić się do niepowodzenia procedury.
SŁOWA KLUCZOWE
Przezwyrostkowa perforująca zmiana okołowierzchołkowa, apiko-marginalny ubytek kostny [MC1], przetoka, mikrochirurgia endodontyczna, sterowana regeneracja tkanek (GTR)