October 16, 2023
J Endod Microsurg. 2023;2:2-23.
Under a Creative Commons license
HOW TO CITE THIS ARTICLE (AMA Referencing)
Tkachenko O, Volokitin A. A clinical case of endodontic microsurgery with a histological diagnosis of an apical scar. J Endod Microsurg. 2023;2:2-23. https://doi.org/10.23999/jem.2023.2.2
NATIONAL REPOSITORY OF ACADEMIC TEXTS
https://nrat.ukrintei.ua/en/searchdoc/2023U000306/
SUMMARY
An apical scar is a rare healing reaction that sometimes occurs when periapical pathology destroys the vestibular and oral cortical plates. Radiographically, this appears as periapical radiolucency and can be mistaken for endodontic pathology or other lesions. The presented clinical case in a 31-year-old female patient shows this well. Based on clinical and imaging (radiography and cone-beam computed tomography [CBCT]) assessment with biopsy, the diagnosis was confirmed. X-ray and CBCT before and 1 year and 6 months after the microsurgery are compared. The multiple detailed intraoperative endodontic microsurgery and histopathology photographs are presented and described; the literature data are analyzed. In this report, the root canal transportation with perforation of the vestibular wall of the root in the area of the tooth 12 is also presented and its management highlighted.
INTRODUCTION
The main criterion for success in conservative root canal therapy performed on a tooth with a radiolucent area in the periapical region is a complete bone regeneration of the region, with re-establishment of the lamina dura and periodontal membrane [1]. There is evidence (Penick, 1961; Bhaskar, 1966; Seltzer and colleagues, 1967; Nair and colleagues, 1999) [1-4] that unresolved periapical radiolucencies may occasionally be due to healing of the lesion by scar tissue that may be misdiagnosed as a radiographic sign of failed endodontic treatment [5].
According to American Association of Endodontists (AAE) Glossary of Endodontic Terms (2020), apical scar is a dense collagenous connective tissue in the bone at or near the apex of a tooth with a distinctive radiolucent presentation [6]. Another scientific source (Lee and colleagues, 2021) [7] gives such a definition: Periapical scar is a reparative response to a periapical inflammatory lesion with the formation of dense fibrous collagenous tissue instead of normal alveolar bone after an appropriate endodontic treatment/retreatment with or without periapical surgery.
These fibrous (periapical) scars occur most frequently when both the facial and lingual cortical plates have been lost [8].
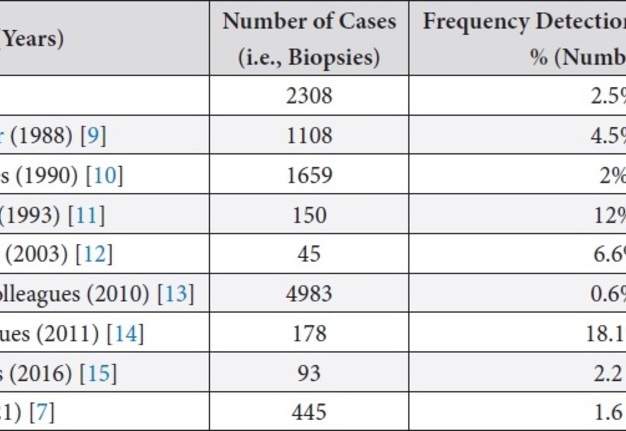
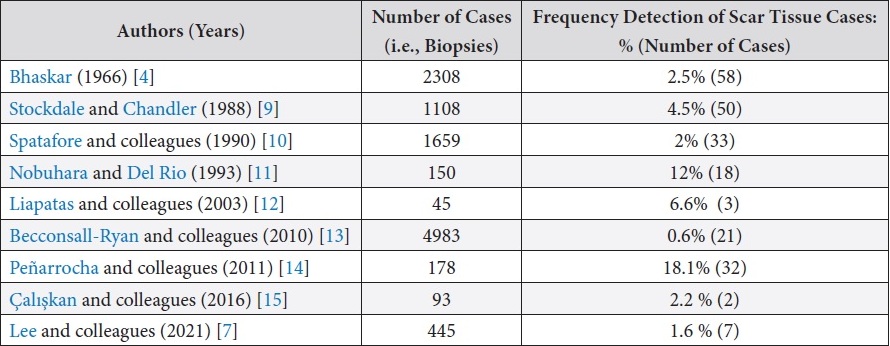
Scientific articles that included histological studies of periapical lesions were analyzed. Table 1 shows the frequency of detection of scar tissue.
TABLE 1. Frequency of Detection of Scar Tissue according to Publications.
It is still not clear why some inflammatory periapical lesions heal with the regeneration of new alveolar bone but others repair with the formation of a fibrous scar tissue after an appropriate endodontic therapy. The regeneration of new alveolar bone in a jaw bone defect needs the undifferentiated mesenchymal stem cells and the induction factors (such as bone morphogenetic proteins) or requires the osteoblasts that migrate from the adjacent healthy periosteum or endosteum directly and some bone growth factors that stimulate osteoblasts to proliferate [16, 17]. The lack of the adjacent healthy periosteum or endosteum to provide the bone forming cells (osteoblasts) may result in a defective healing with the formation of fibrous scar tissue [7]. The pattern of healing depends on several factors, two of which are decisive: the regenerative potential and the speed with which the tissue cells bordering the defect react. A periapical scar probably develops because precursors of soft connective tissue colonize both the root tip and periapical tissue; this may occur before the appropriate cells, which have the potential to restore various structural components of the apical periodontium, are able to do so [5].
There is a hypothesis that explains why a periapical scar is formed. This hypothesis states that periapical scar formation is caused by bone inhibitory molecular signaling from the epithelial cell rests of Malassez. When these cells are present in teeth with an infected root canal system, a periapical cyst develops, whereas in the case of a treated root canal system infection, periapical inflammation diminishes and periapical lesion heals until the regeneration process reaches the apical part of the tooth where epithelial cell rests of Malassez are present. Cytokines cause rapidly progressive defensive fibroproduction and scar formation, in which osteoblasts cannot differentiate into bone [18].
It was noted that this lesion occurs more often in the maxilla than in mandible, and patients of the fifth decade of life [4].
In general, there is no need to treat this kind of fibrous scar if the clinicians can recognize that the periapical lesion is merely a periapical scar. Therefore, the difficult problem is how to differentiate the rare periapical scar lesion from the more common periapical lesions such as periapical granuloma and radicular cyst. The common clinical and radiographic features of periapical scars would be [7]:
-
The previous endodontic treatment/retreatment shows an adequate root canal filling.
-
The previous periapical surgery is well performed with a proper retrograde filling.
-
The involved tooth is free from any symptom and sign.
-
The involved tooth has no evidence of root fracture and healthy periodontium except the periapical radiolucency.
-
The well-defined periapical radiolucent lesion has persisted without a significant change of its size for a long period of time.
The purpose of this case report is to provide education and awareness regarding apical scar in traumatized anterior upper teeth based on the case in a 31-year-old female. Pre- and post-operative clinical view, x-ray, cone-beam computed tomography (CBCT), intraoperative endodontic microsurgery stages, and histopathology photographs will be analyzed.
CASE REPORT
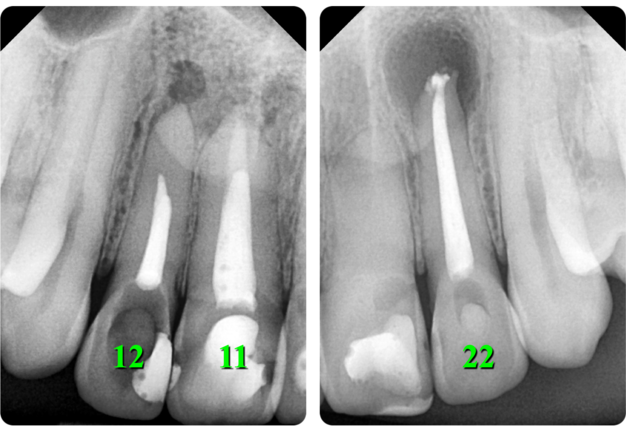
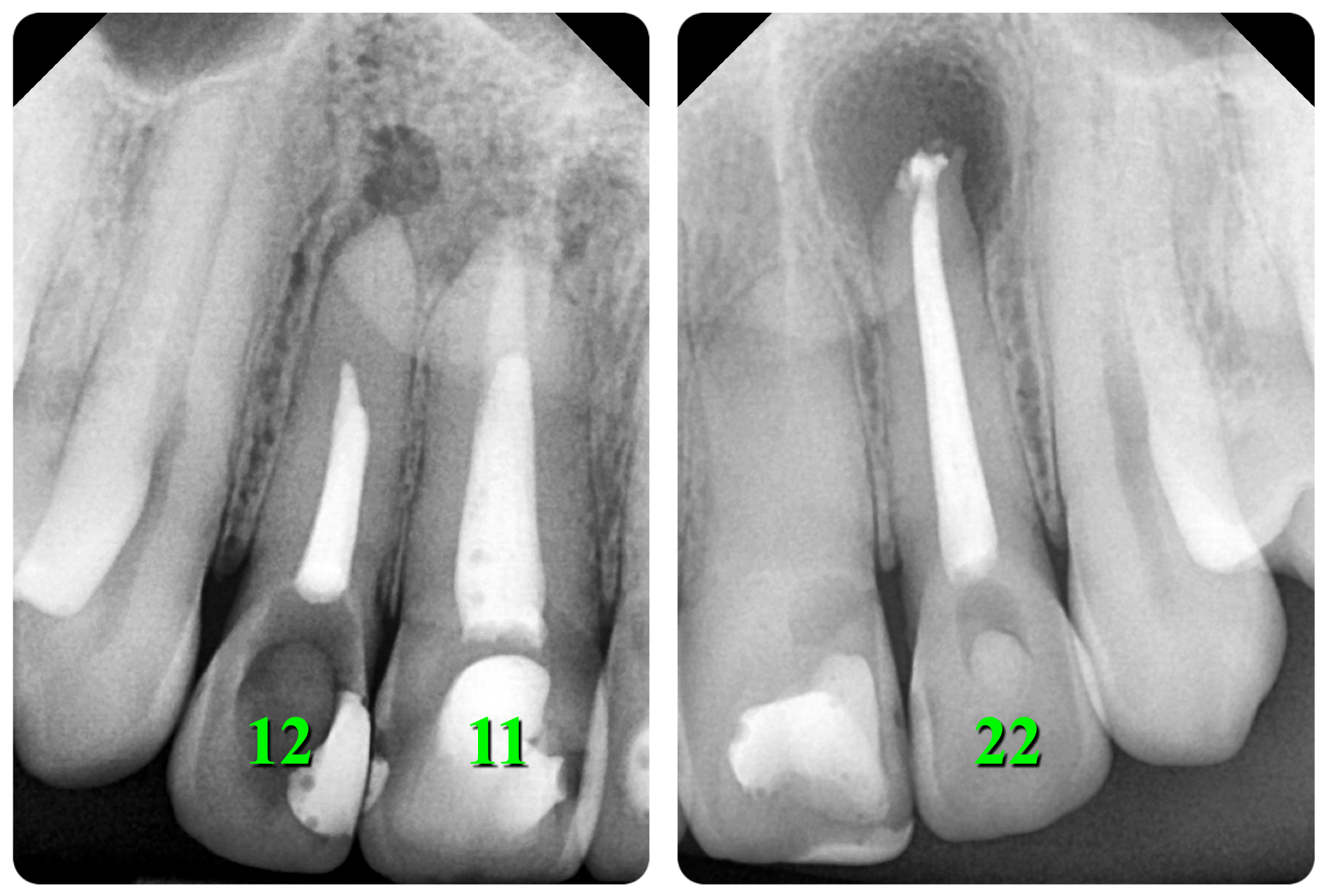
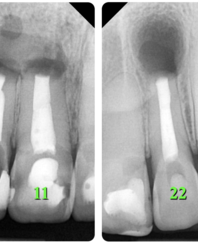
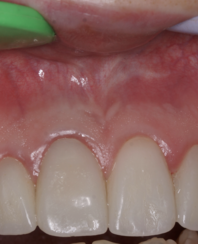
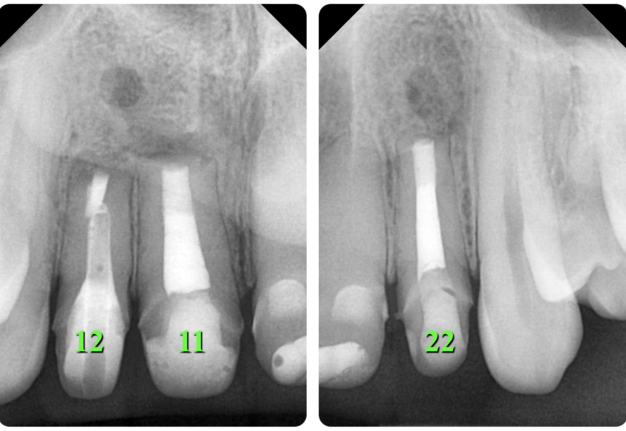
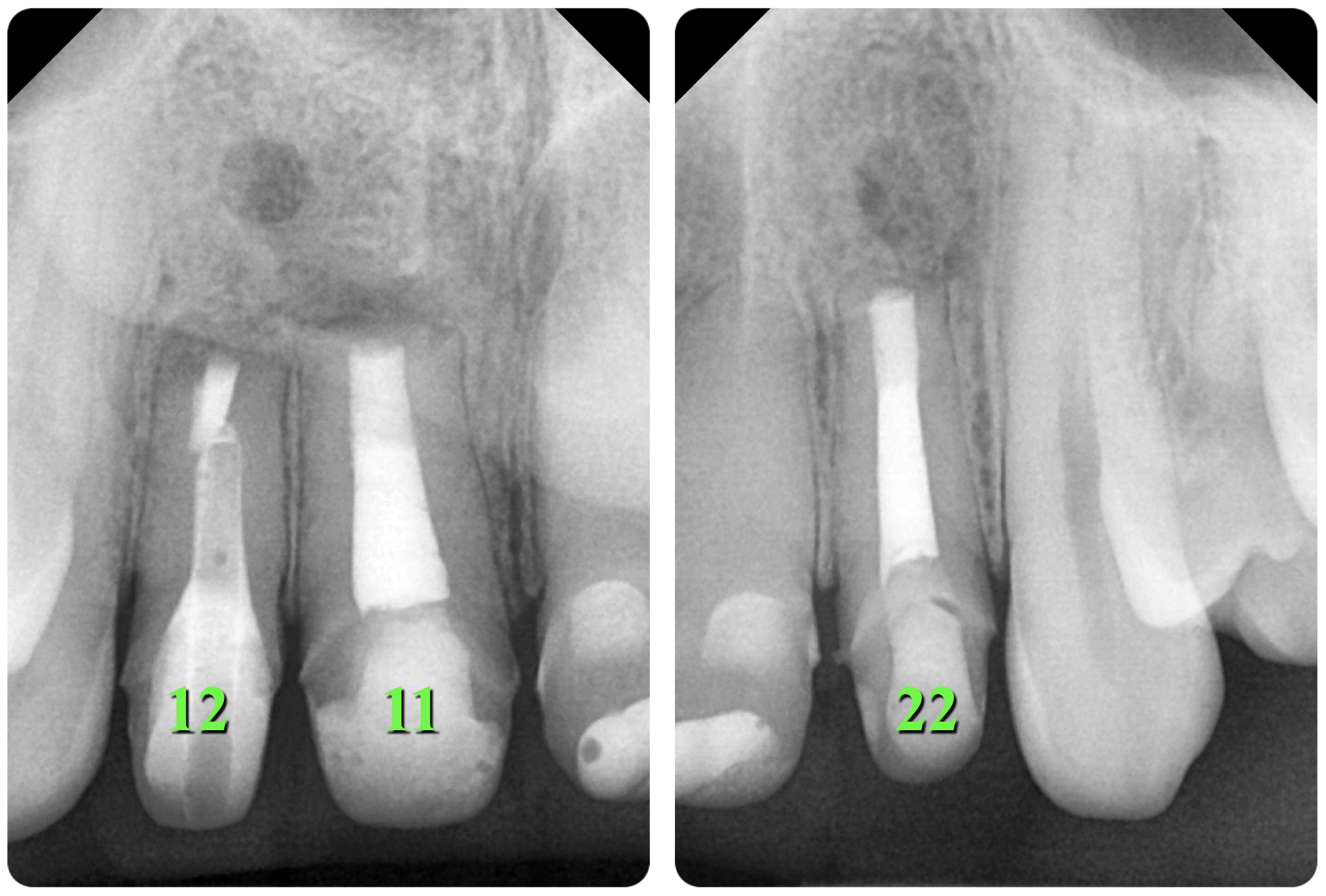
A 31-year-old female referred for endodontic microsurgery of the teeth 12, 11, and 22 (Fig 1).The patient did not notice general somatic pathology. It was known from the anamnesis that in 2000 she was hospitalized (department of oral and maxillofacial surgery) for 7 days due to an injury to the upper front teeth—the extrusive luxation of 12, 11, 21, and 22 teeth were diagnosed. A splint was put on the teeth for 1 week.
According to the patient, root canals in teeth 12, 11, and 22 were first treated around 2017 for pulpitis. At the stage of root canal treatment, temporary fillings were placed. The patient stayed with temporary fillings for several months, as it was not possible to continue the treatment. The patient notes that during this time some fillings fell out and the teeth were open for several months. Retreatment of root canals in the teeth 12, 21, and 22 was carried out in 2019 by a general dentist.
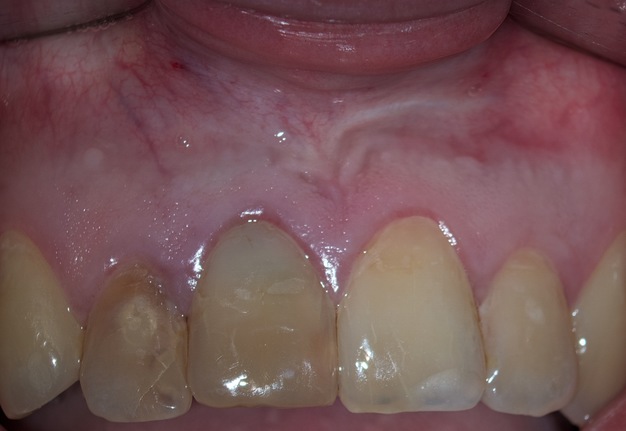
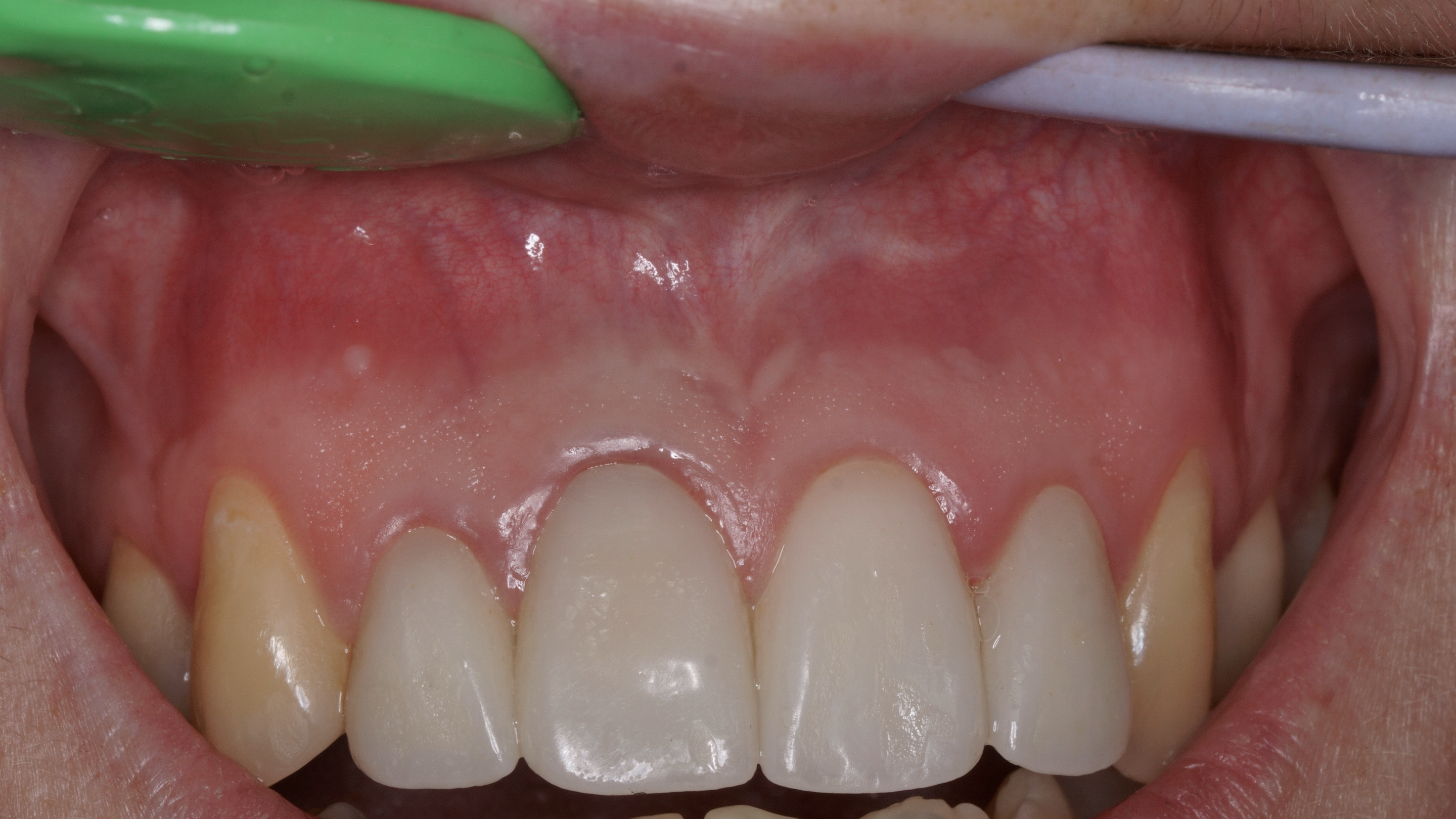
Clinically, during checkup and examination, teeth 12 (upper right lateral incisor) and 11 (upper right central incisor) were discolored (Fig 2).
Photopolymer fillings are present on the palatal surface of teeth 12, 11, 21, and 22. Percussion of teeth 12, 11, 21, and 22 was negative. Palpation is negative in the area of the transitional fold of the mucous membrane from the vestibular and palatal side. There is a scar in the area of the transitional fold of the mucous membrane on the vestibular surface in the area of the teeth 12, 11, 21, and 22. Periodontal examination is unremarkable, there is no mobility of the teeth, the regional lymph nodes on the right and left sides are not enlarged, not painful, mobile and not fused to the surrounding tissues. Mouth opening unchanged. Movements of mandible without peculiarities.
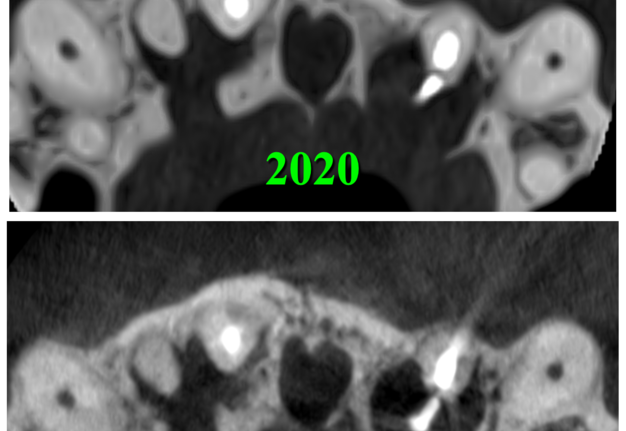
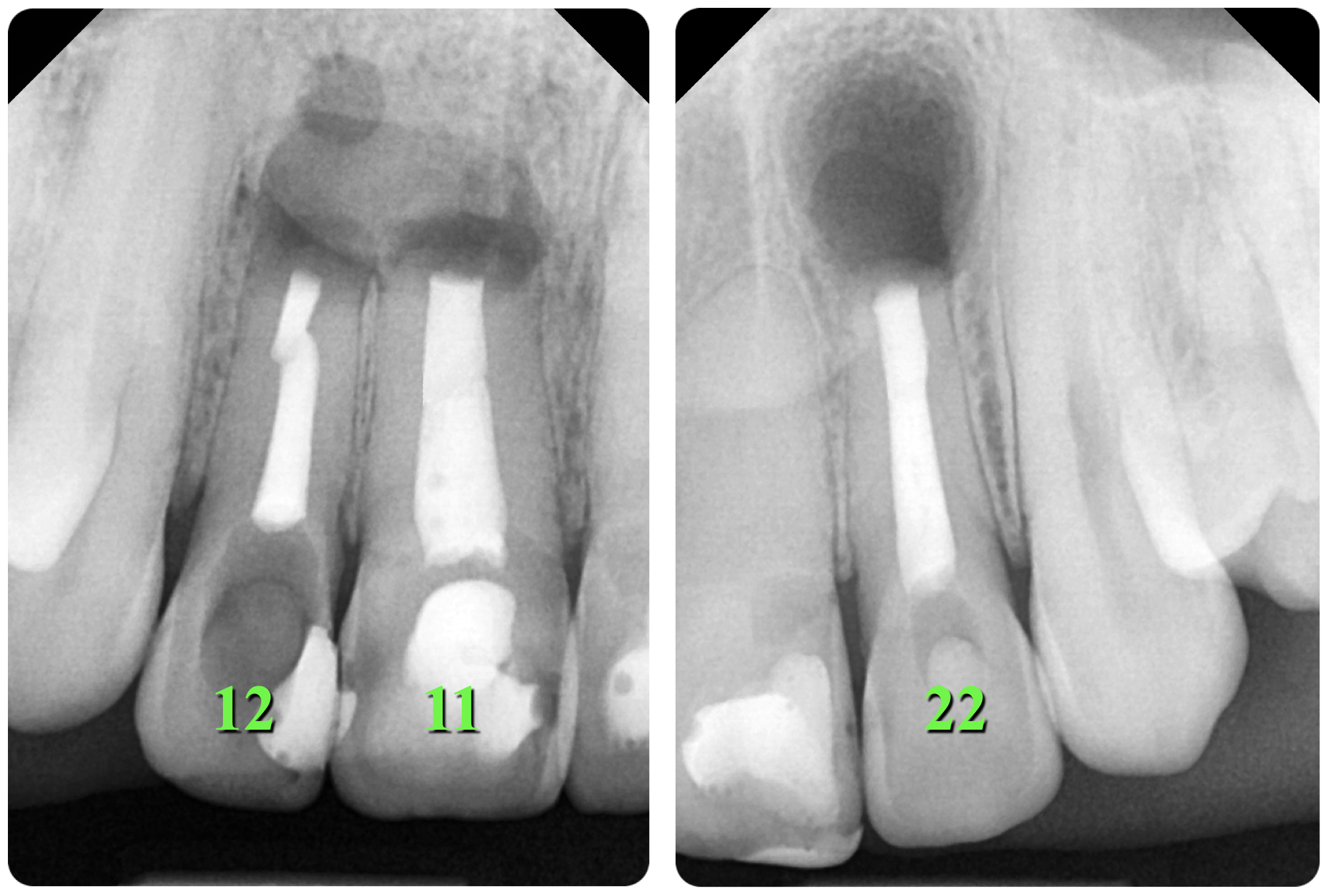
After the retreatment of a root canal of the tooth 22, the patient periodically (several times) noticed a dull aching pain. Comparing the CBCT scans for 2020 and 2021, we can say that there was no increase in periapical radiolucency/rarefaction in areas of the teeth 12, 11, and 22 (Fig 3).
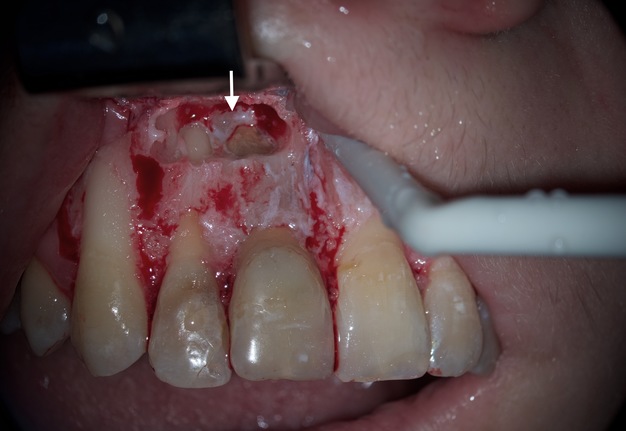
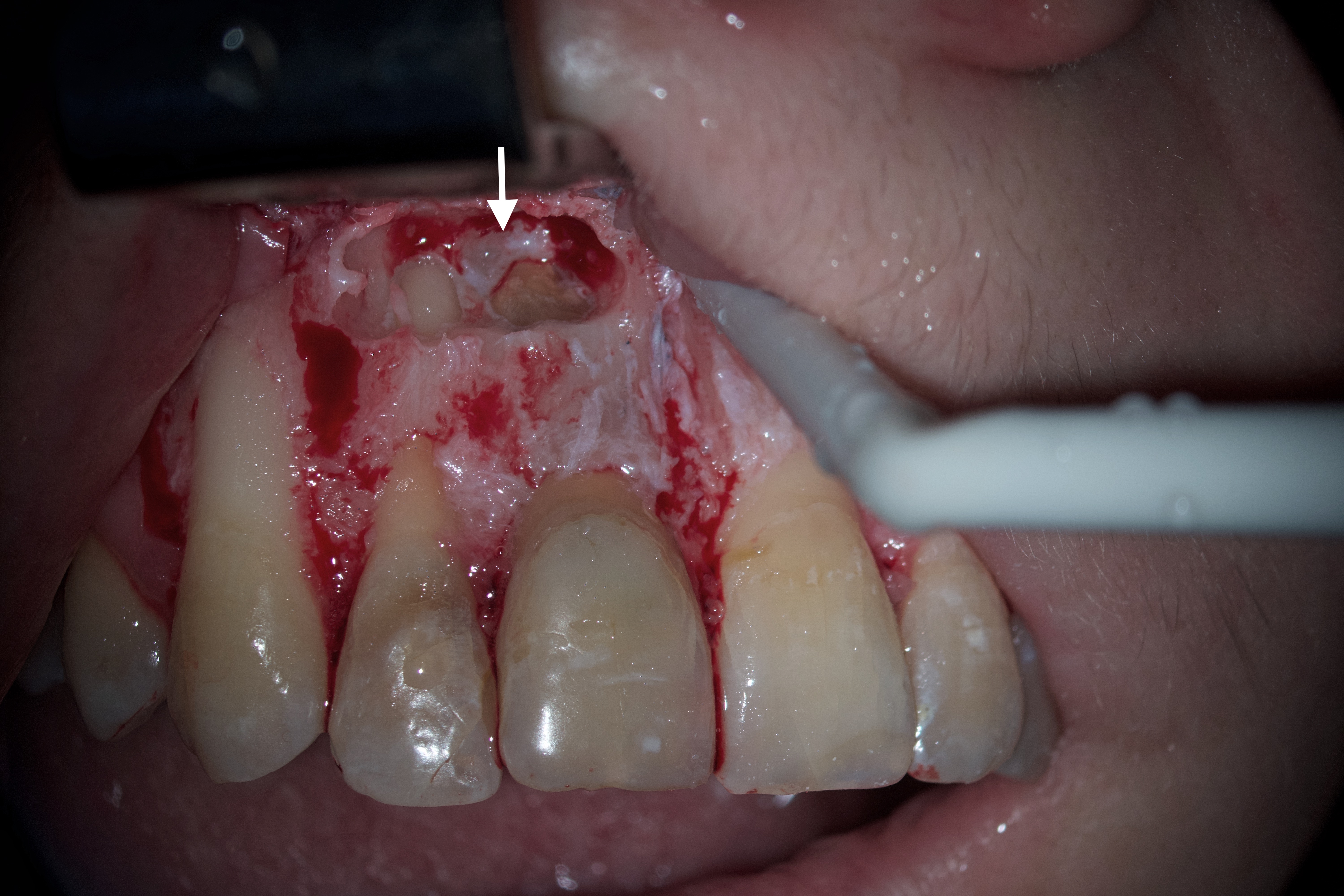
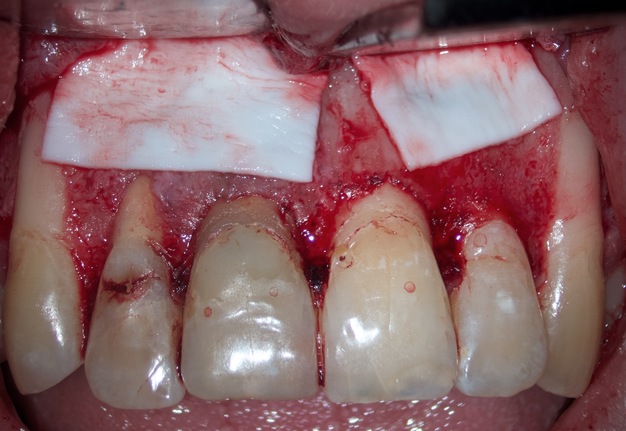
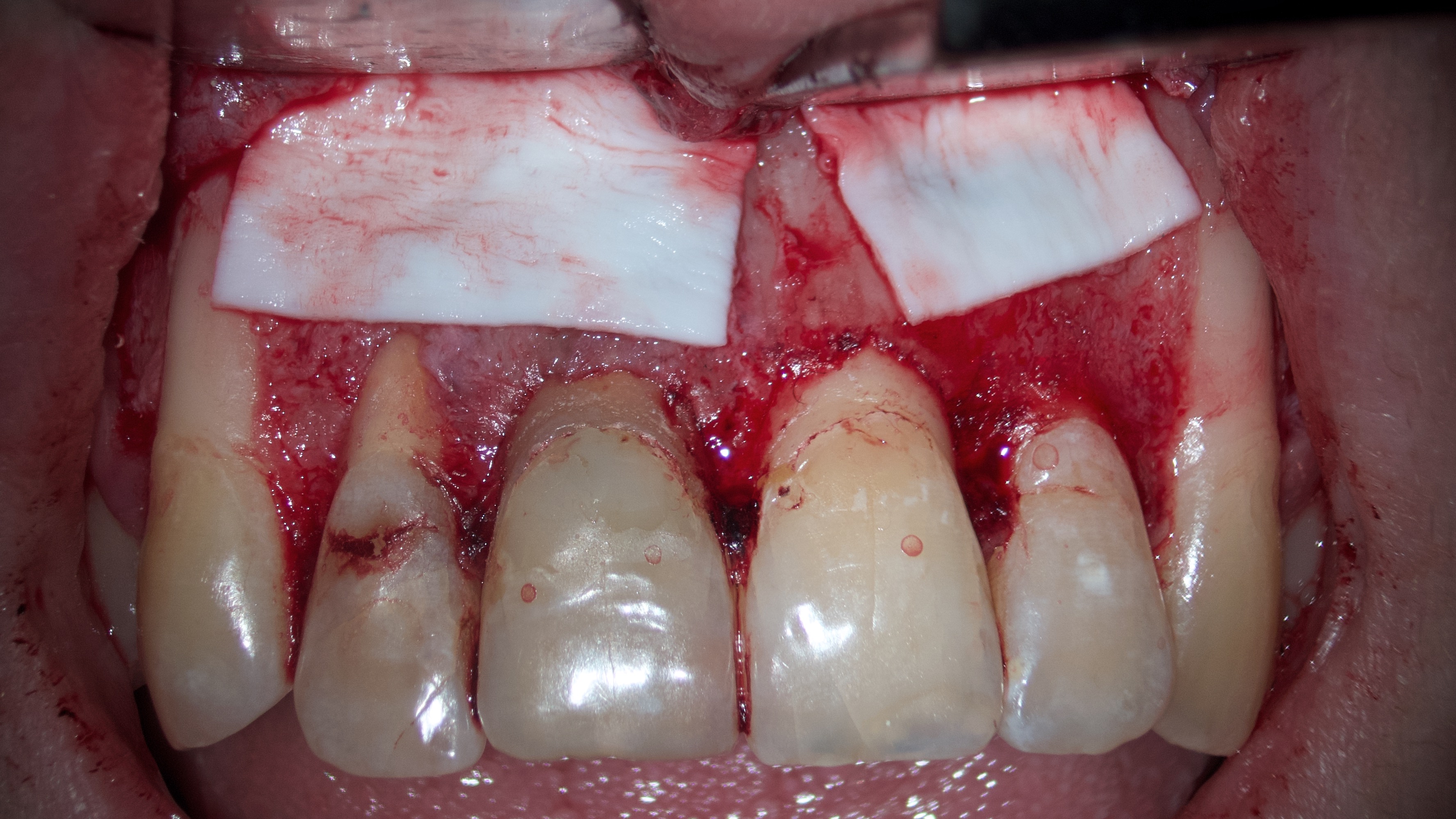
Endodontic microsurgery was planned to save the teeth 12 (upper right lateral incisor), 11 (upper right central incisor), and 22 (upper left lateral incisor). All manipulations were performed by the experienced doctor (O.B.T.: 9 years of work with operating microscope) under the control of an OPMI® pico (Carl Zeiss, Gottingen, Germany) operating microscope. Professional oral hygiene and antiseptic treatment of the oral cavity with 0.12% chlorhexidine solution (Chlorhexidine Denta, Hrybyk A.I. Individual Entrepreneur on the production premises of Pharmaceutical Factory LLC, Ivano-Frankivsk, Ukraine) were performed. The incision line was treated with 5% iodine solution. Infiltration anesthesia with 2.5 ml UbistesinTM forte (4% articaine with 1:100,000 adrenaline [3MTM Deutschland GmbH, Neuss, Germany]) was performed. In the area of the neck of teeth 12 and 22, the gingivectomy was performed to improve aesthetics (wishes of the referring stomatologist). Intrasulcular incision was made. A full-layer muco-periosteal flap was elevated and an osteotomy with a Lindemann burr H162 (Komet Dental, Gebr Brasseler GmbH & Co KG, Lemgo, Germany) was performed. In the area of the defect between the teeth 12 and 11, a pathologically changed tissue that had a dense, fibrous consistency and a white color was noted (Fig 4).
Resection of the root apices of the teeth 12, 11, and 22 were performed by 3 mm (Fig 5) with a diamond bur (a green one according to ISO).
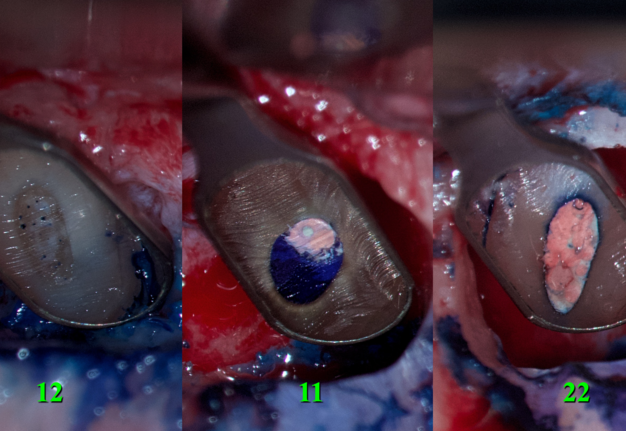
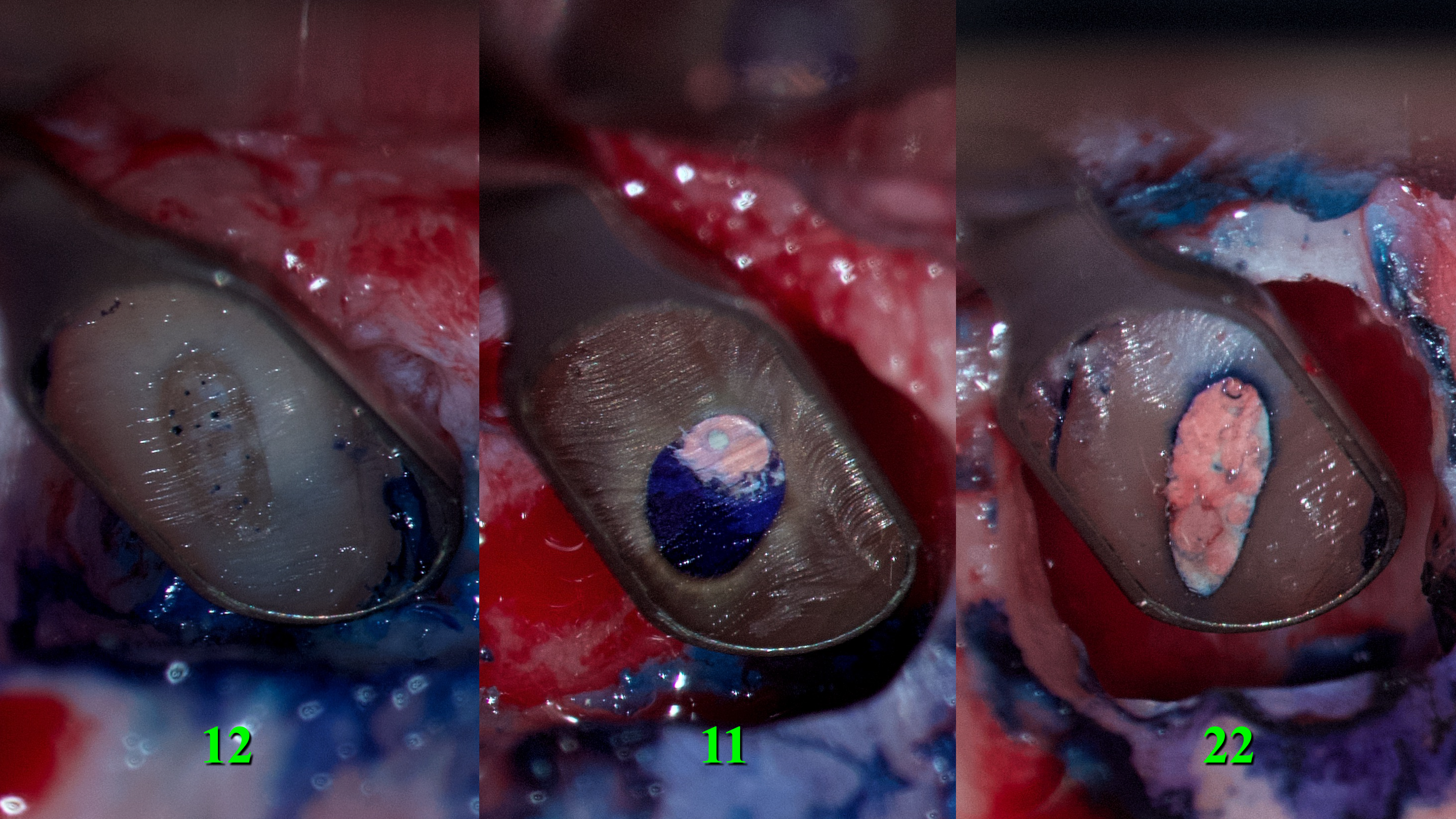
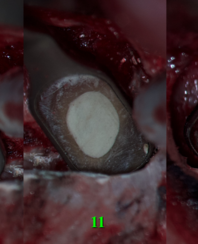
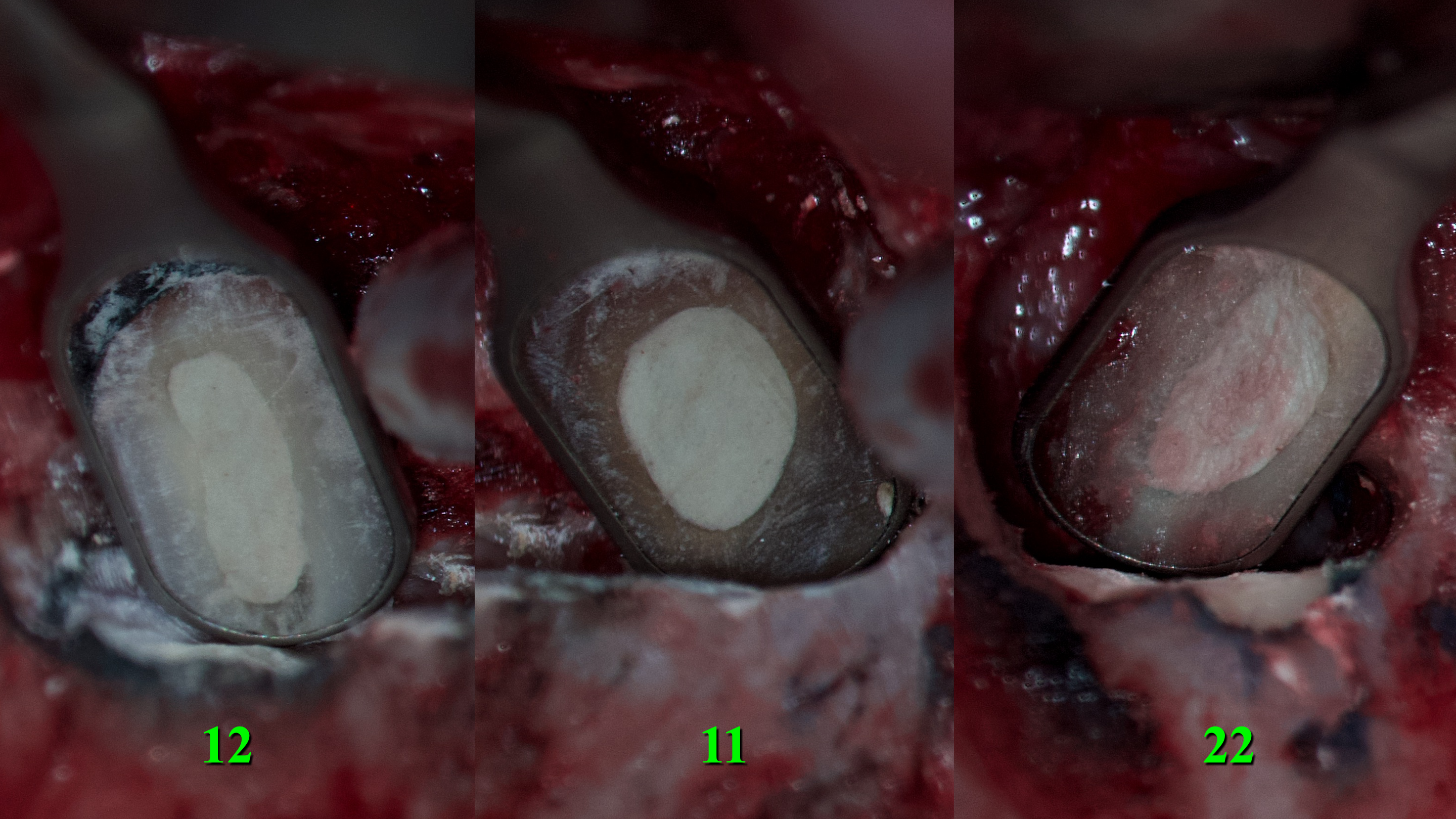
The resected root surfaces were polished with diamond bur (yellow according to ISO). Hemostasis was performed with epinephrine pellets (RacelletTM Cotton Pellets size 3 [hemostatic cotton pallets with epinephrine], Pascal International, Inc, Bellevue, WA, USA). Next, a 1% aqueous solution of methylene blue dye (10mL Bottle of Methylene Blue, Vista-BLUETM, Inter-Med, Inc, Racine, WI, USA) was applied to the resected root surfaces using a micro-applicator brushes for 10 seconds [19]. With the help of a MEGAmicro, oval 3 × 6 mm, stainless steel, REF 6232 (Hahnenkratt E. GmbH, Königsbach-Stein, Germany) retrograde mirror, an examination of the resected root surfaces was carried out (Fig 6).
FIGURE 6. With the help of a MEGAmicro, oval 3 × 6 mm, stainless steel, REF 6232 (Hahnenkratt E. GmbH, Königsbach-Stein, Germany) retrograde mirror, the examination of the resected root surfaces was carried out. 12, upper right lateral incisor; 11, upper right central incisor; 22, upper left lateral incisor.
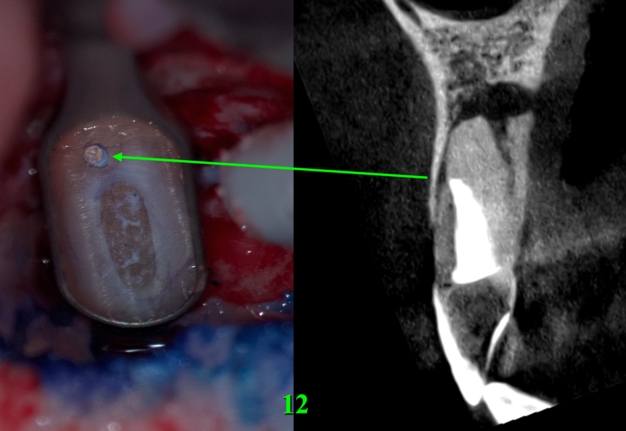
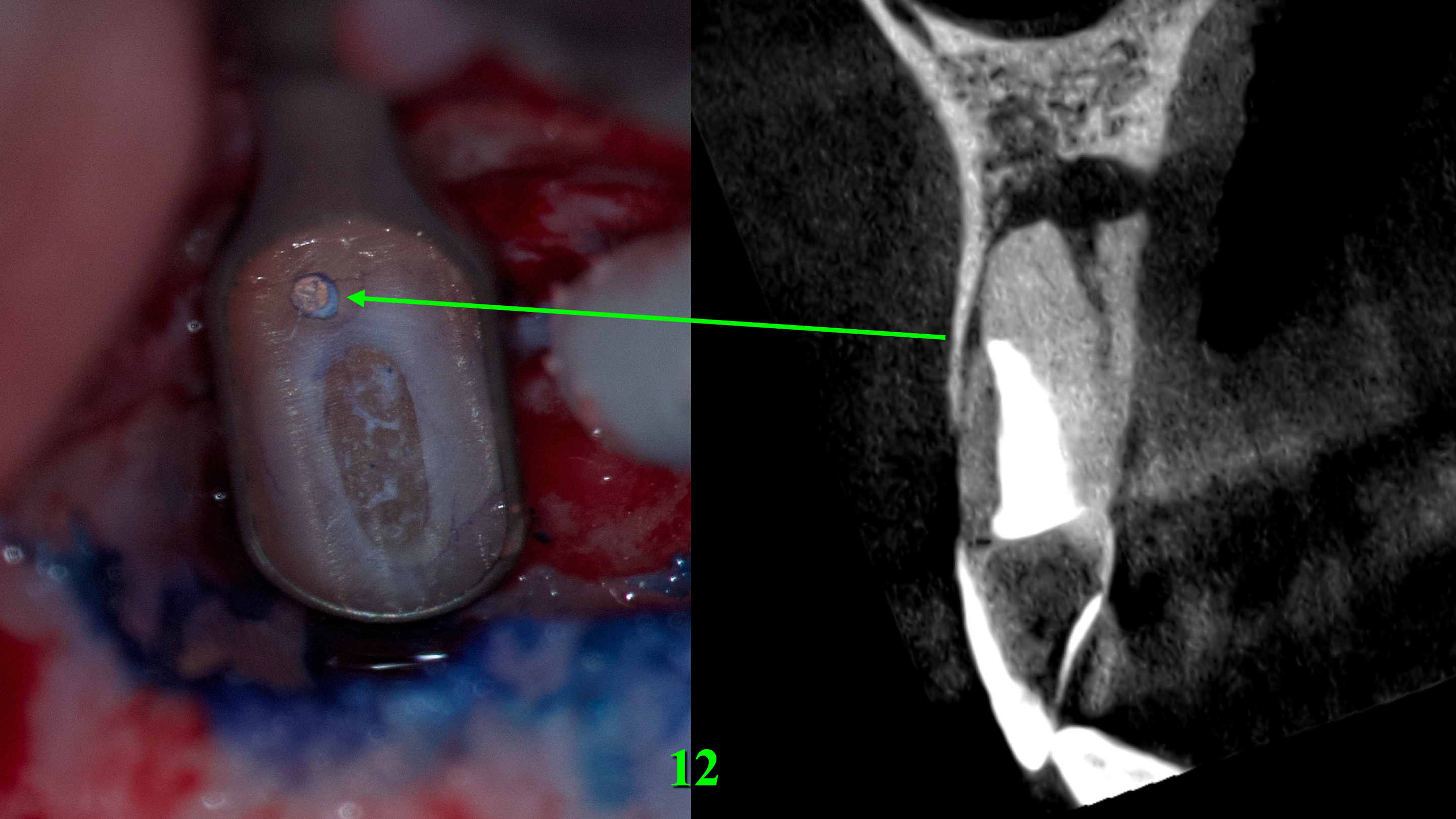
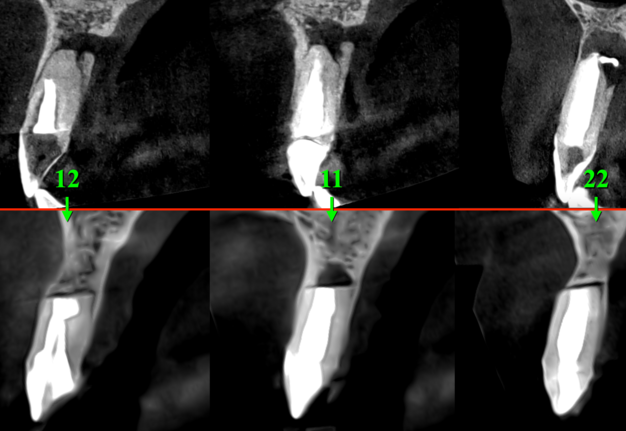
Looking at the resected surface of tooth 12, one can see signs of sclerosing of the lumen of the root canal, namely calcifying metamorphosis that develops precisely as a result of trauma [5]. The blue dots are microtubules that contain the remains of necrotized pulp. On the resected surface of teeth 11 and 22, it can be seen that methylene blue painted over the perimeter of the filling material in the root canal, which may indicate the presence of gaps with voids and inadequate sealing. Since there is root canal transportation with perforation of the vestibular wall of the root in the area of the tooth which was manifested by slight rarefaction in this place, a decision was made to resect the surface of this root by another 0.5 mm so that there was access to transportation with the possibility of making a retrograde preparation (Fig 7).
FIGURE 7. Since there was root canal transportation (arrow) (A) with perforation of the vestibular wall of the root in the area of the tooth 12 which was manifested by slight radiolucency in this place on a sagittal CBCT scan (B), a decision was made to perform a resection of the surface of this root by another 0.5 mm so that there was access to transportation with the possibility of making a retrograde preparation. 12, upper right lateral incisor.
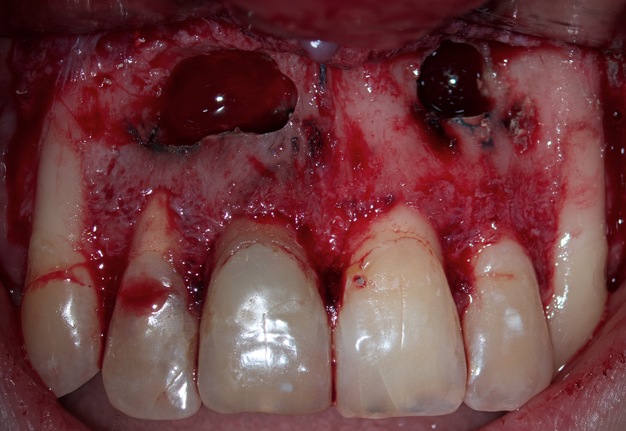
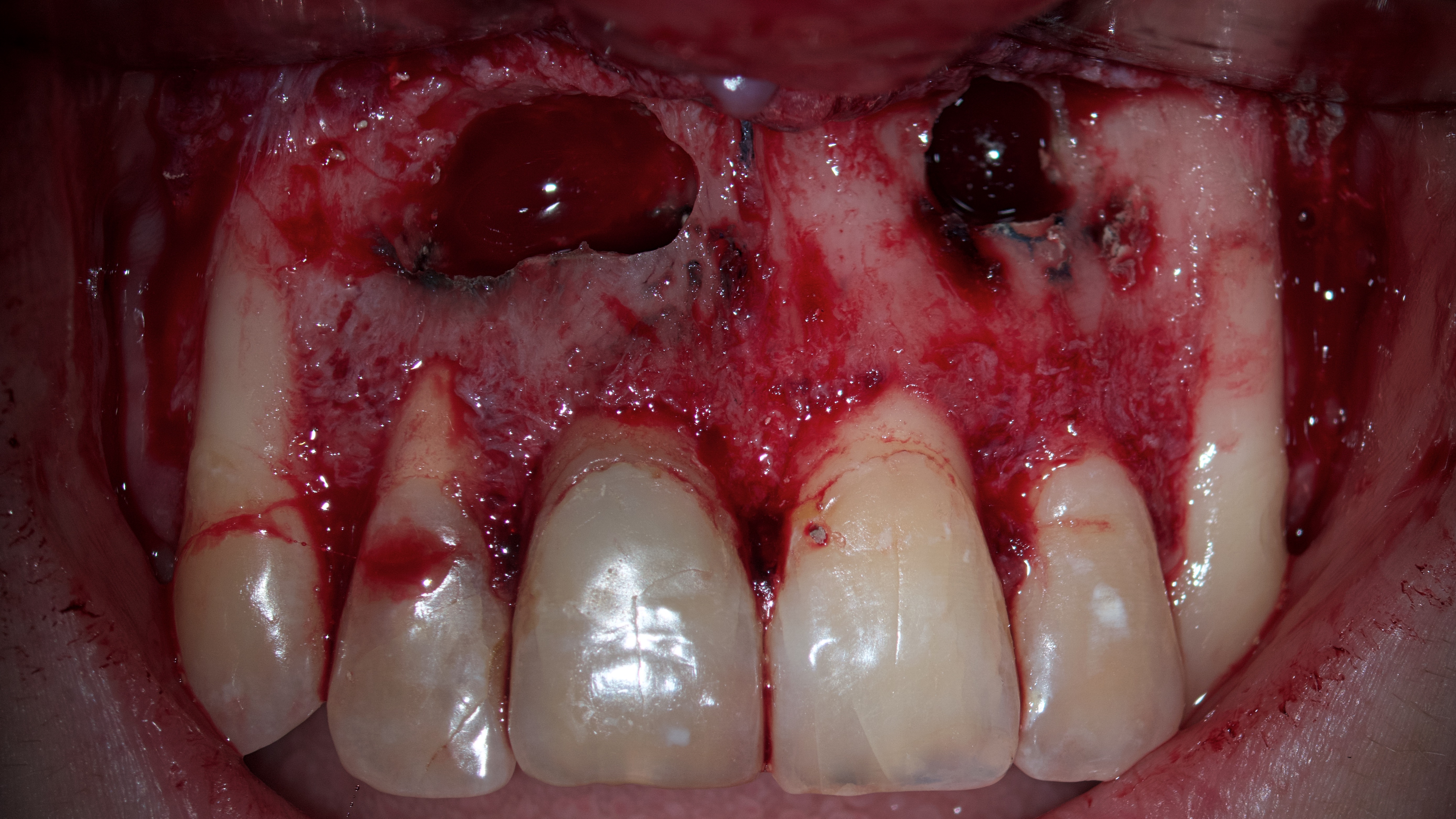
Two incisional biopsies were performed. The first one in the area of teeth 12 and 11. The second is in the area of tooth 22. These specimens were placed in separate tubes of 10% buffered formalin solution and sent for histopathological examination. Next, retrograde preparation of these three teeth was performed with a 3-mm Acteon apical surgery diamond-coated tip (AS3D, 3-mm length, Acteon® Group, Mérignac, France). Retrograde irrigation with 2% chlorhexidine solution (Osteohex, Scientific Production Enterprise Osnova LLC, Kharkiv, Ukraine). Drying with sterile paper points. Retrograde filling with mineral trioxide aggregate (MTA) (Bio MTA+, P.P.H. Cerkamed Wojciech Pawlowski, Stalowa Wola, Poland) (Fig 8).
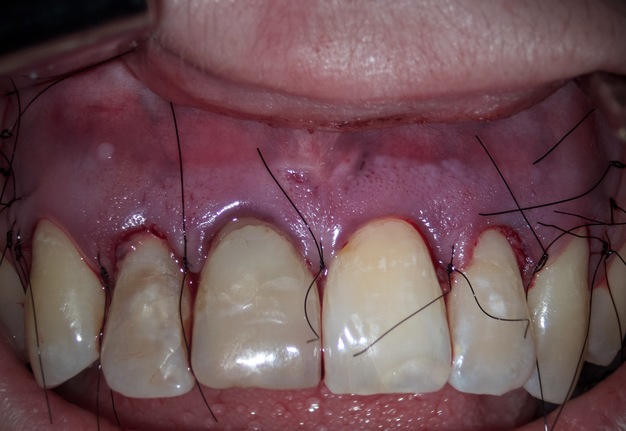
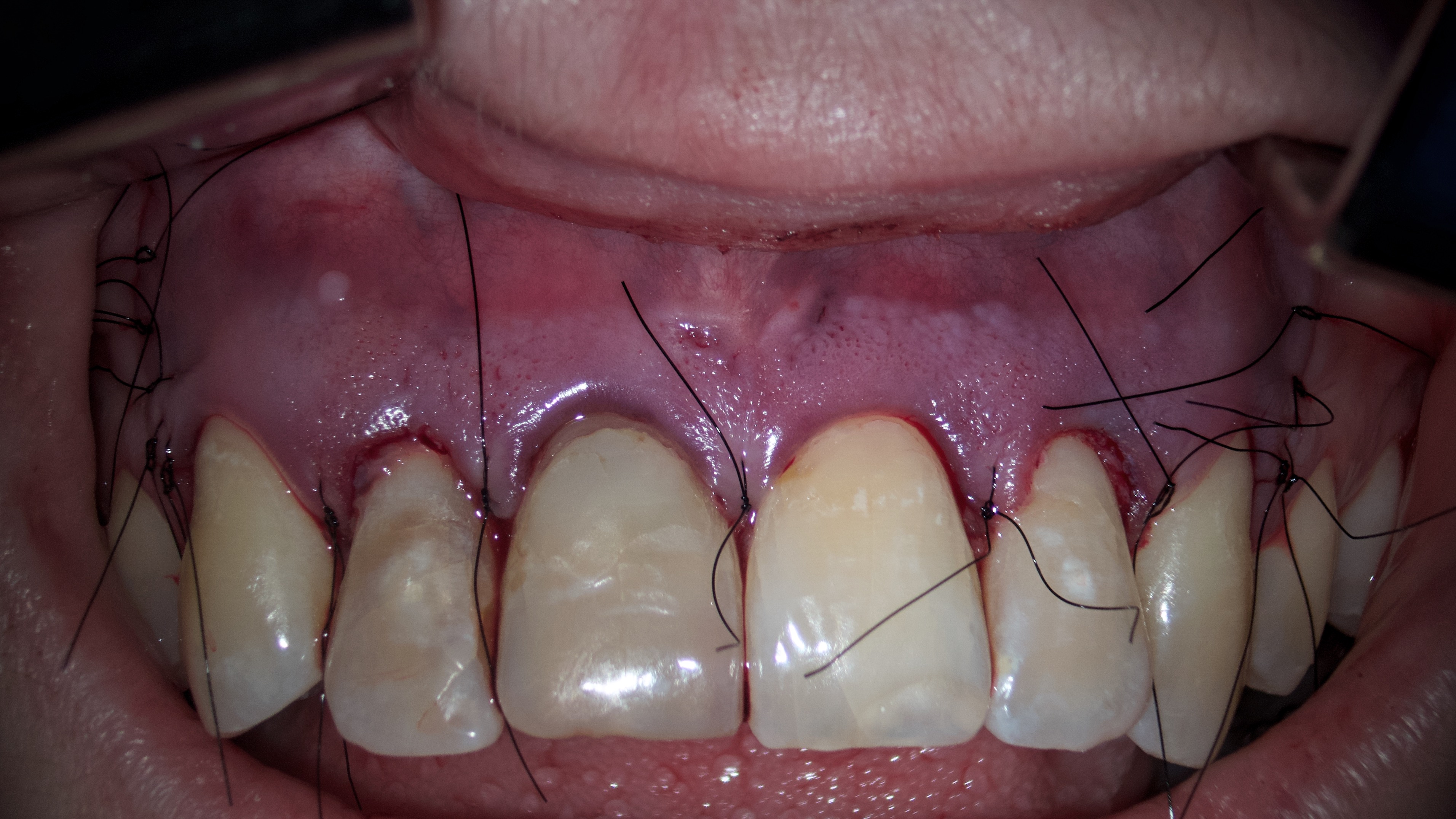
Since there was a through and through bone defect in the area of teeth 12, 11, and 22, the Evolution (OsteoBiol® [collagen resorbable membrane], Tecnoss Dental S.R.L., Torino, Italy) collagen membrane was used (Fig 9). The flap was sutured using nylon 6.0 Nylon (Resorba®, RESORBA Medical GmbH, Nürnberg, Germany) (Fig 10).
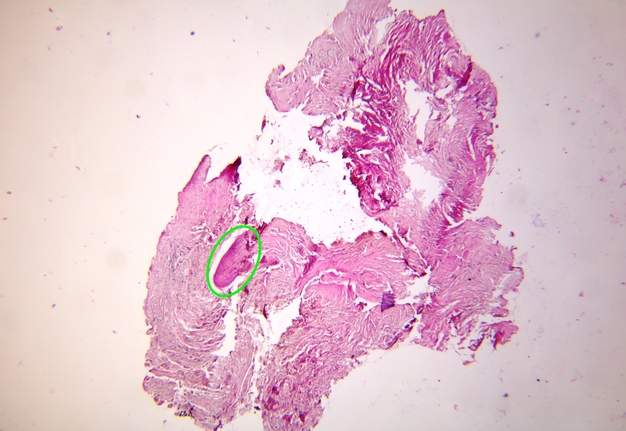
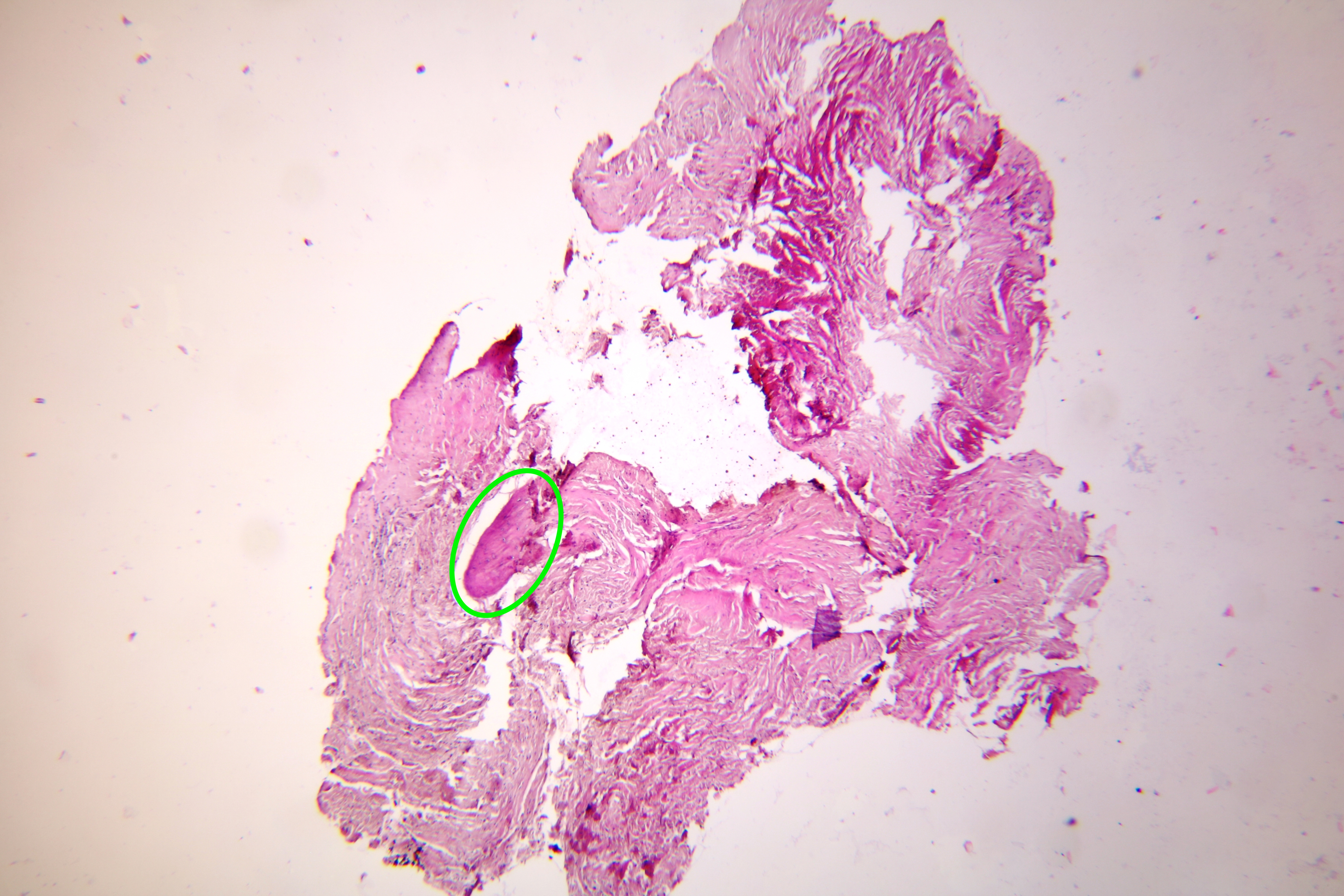
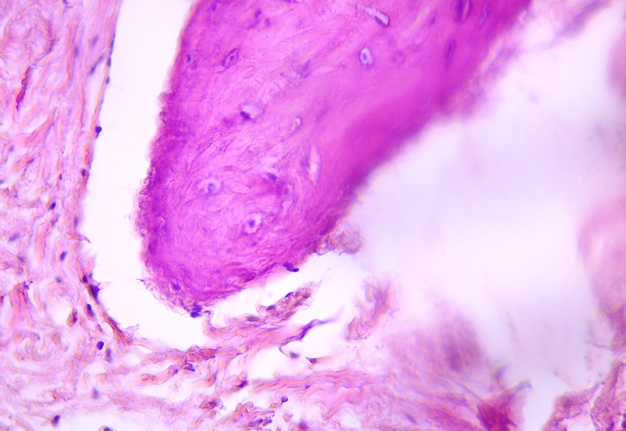
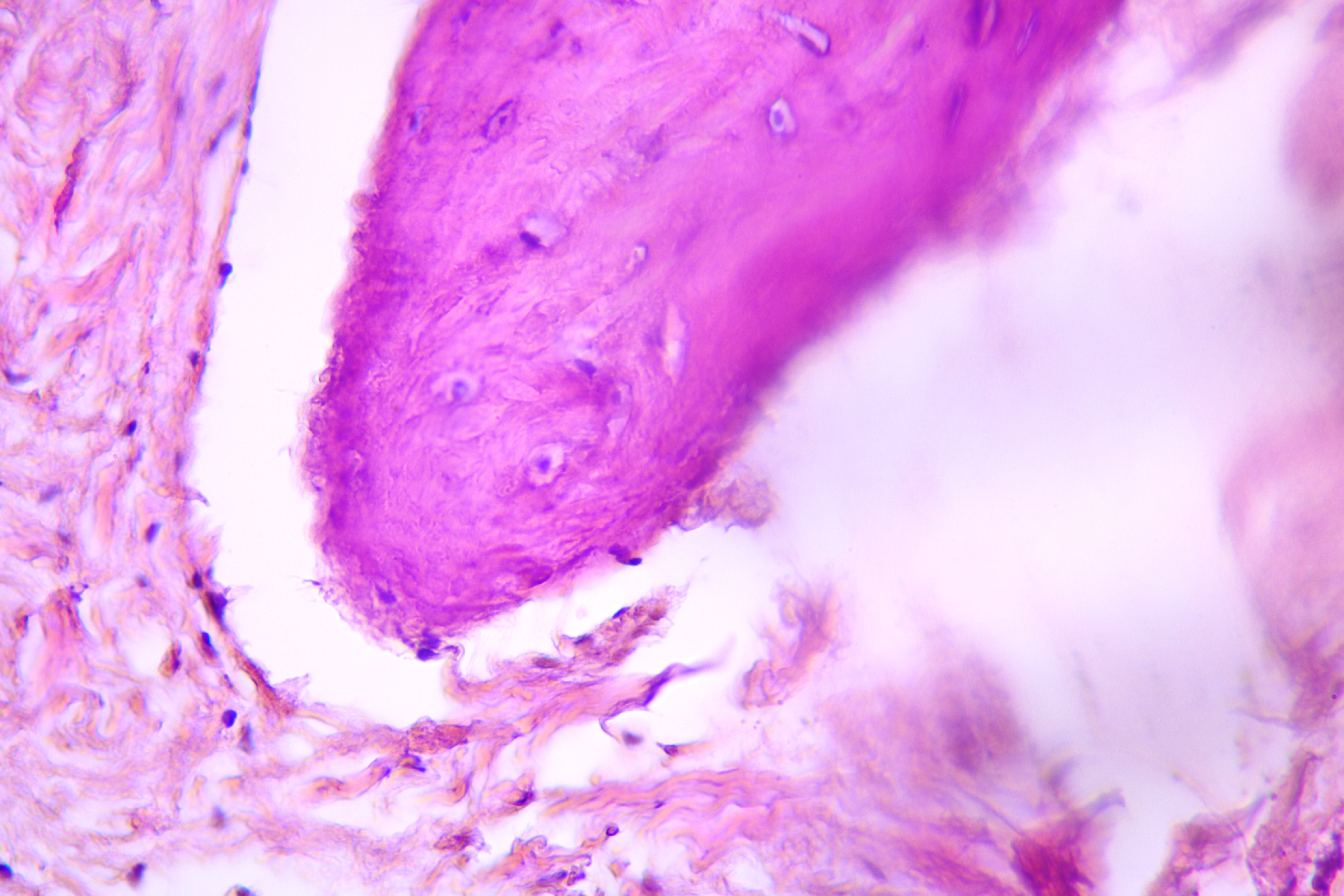
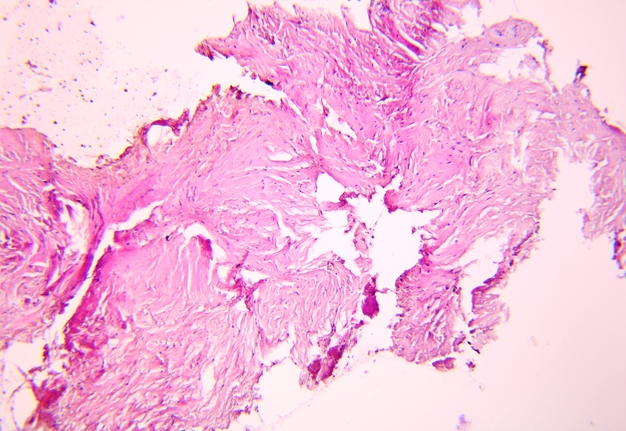
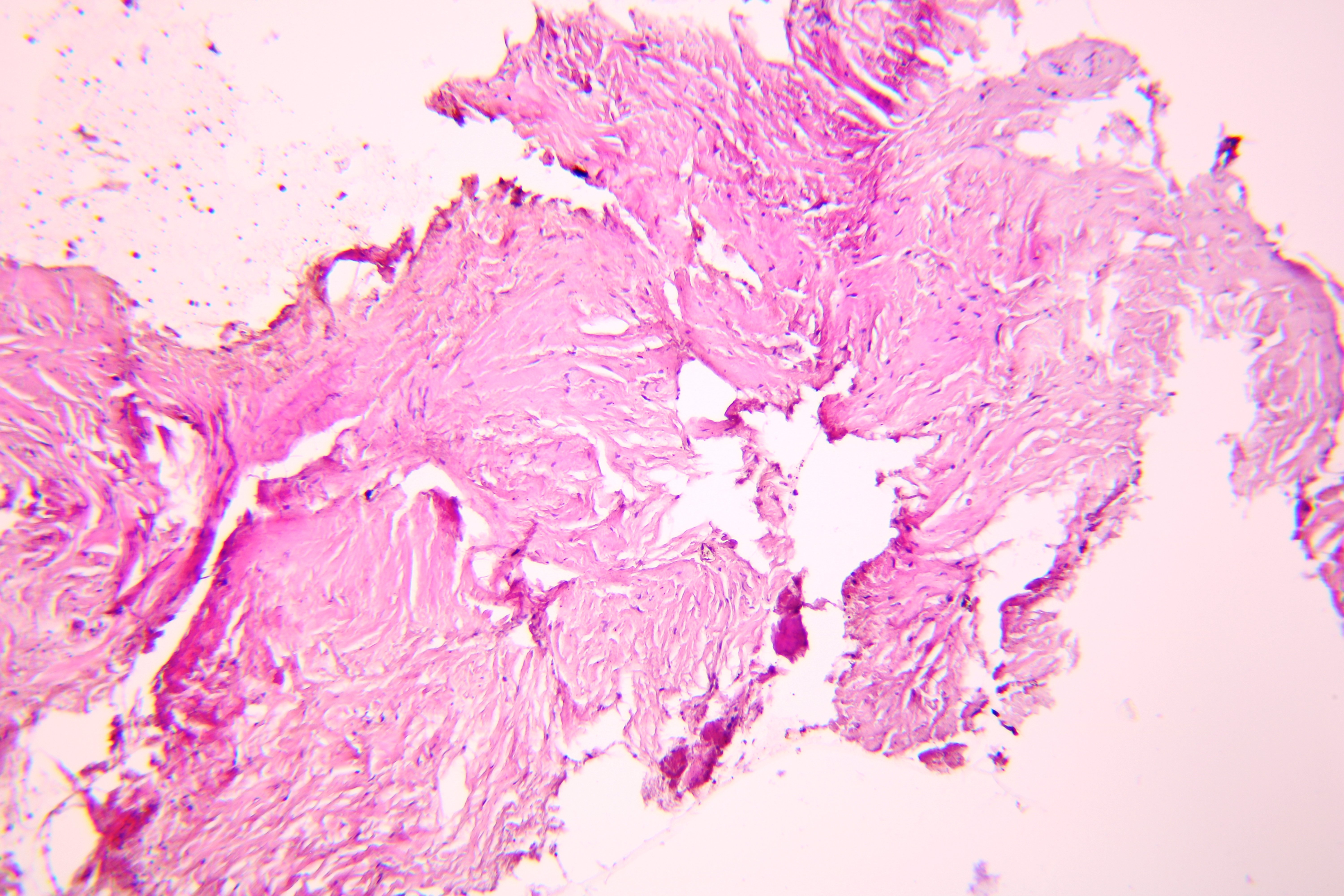
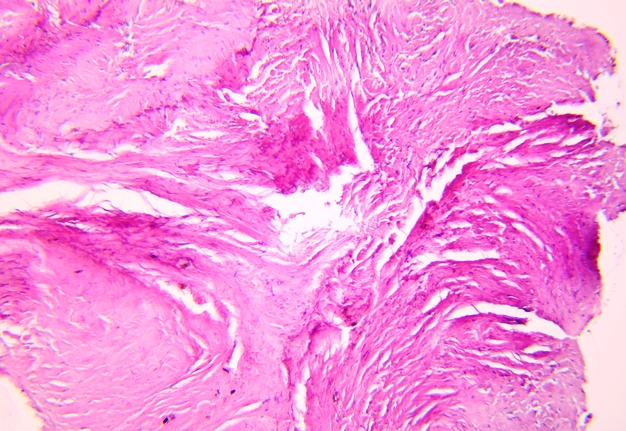
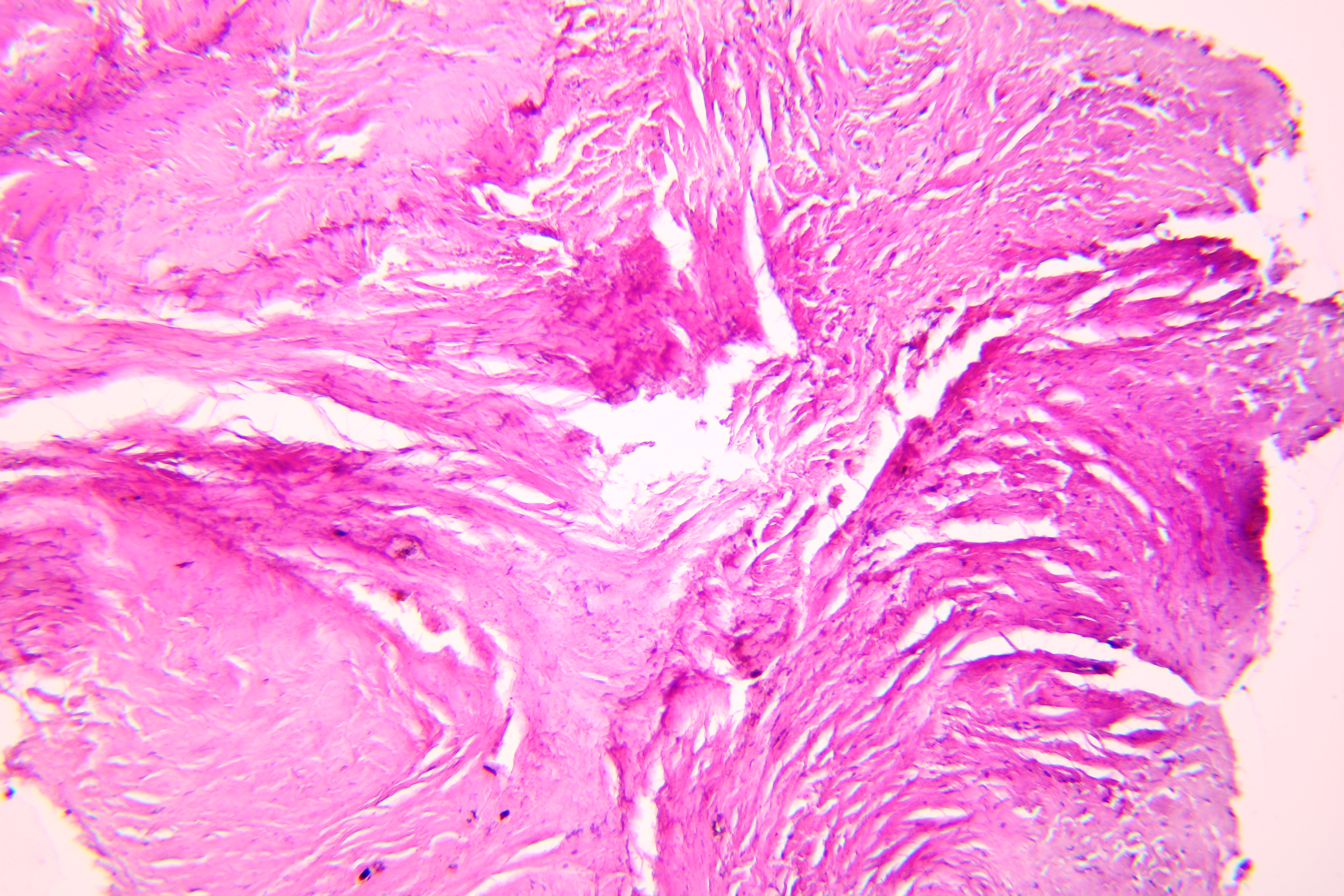
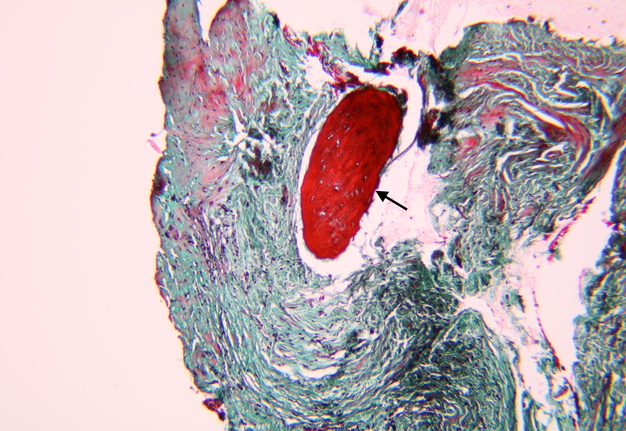
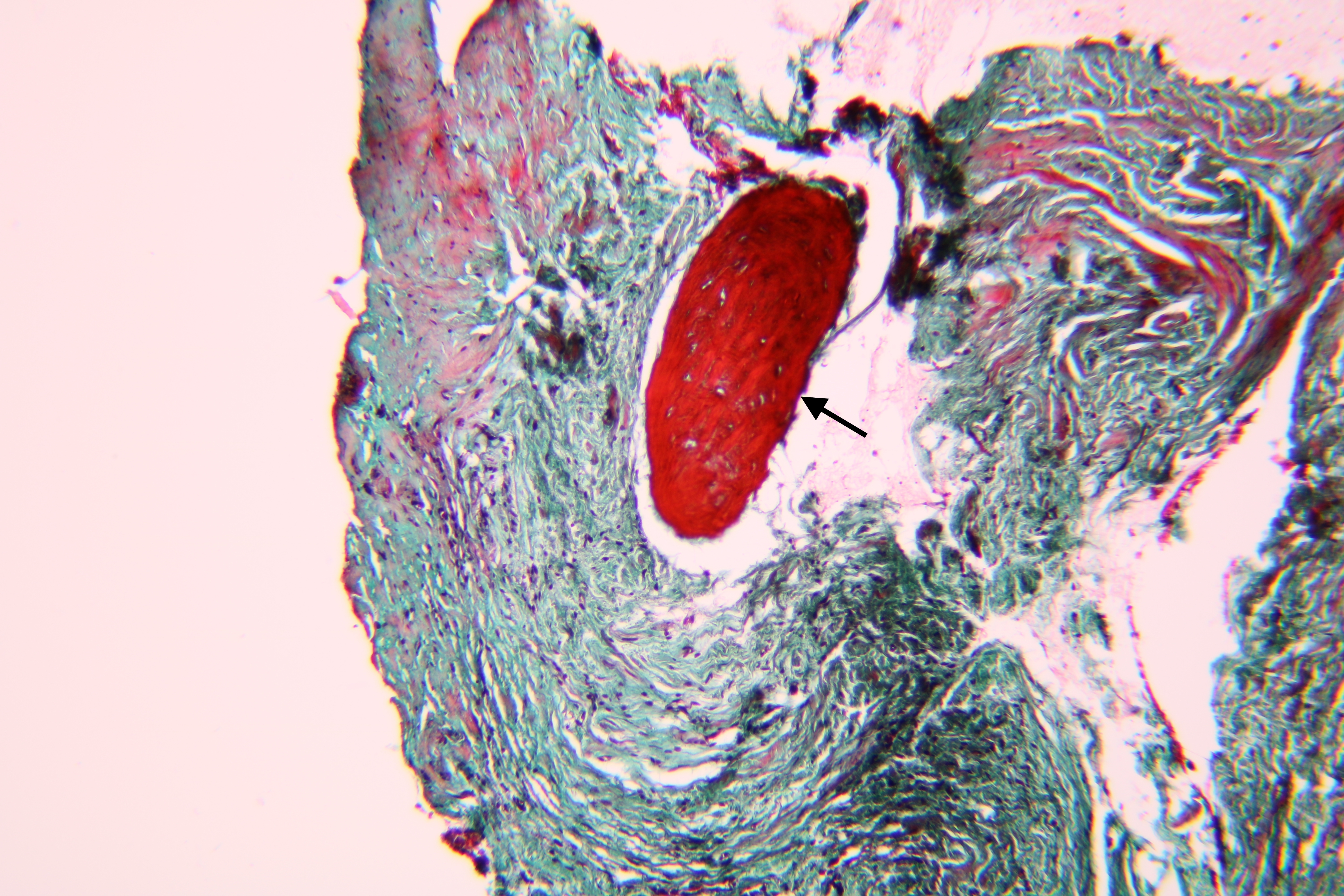
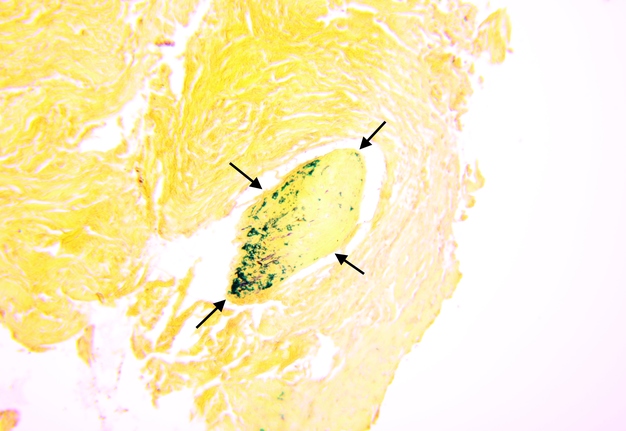
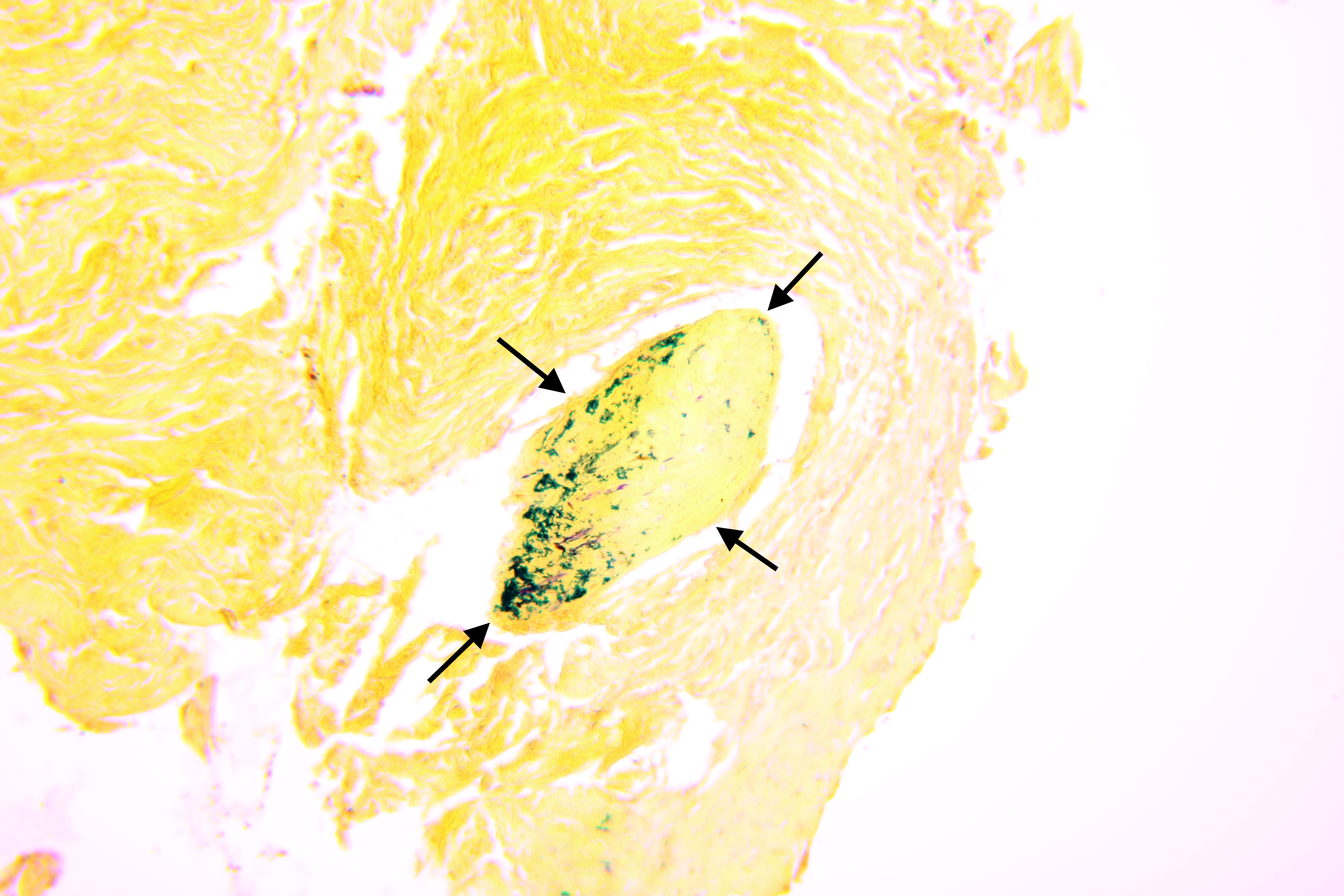
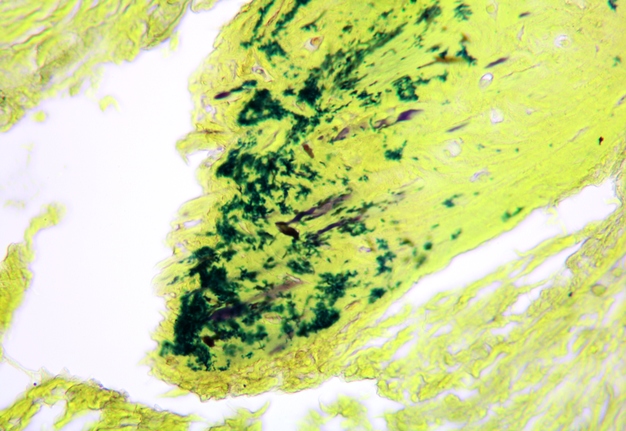
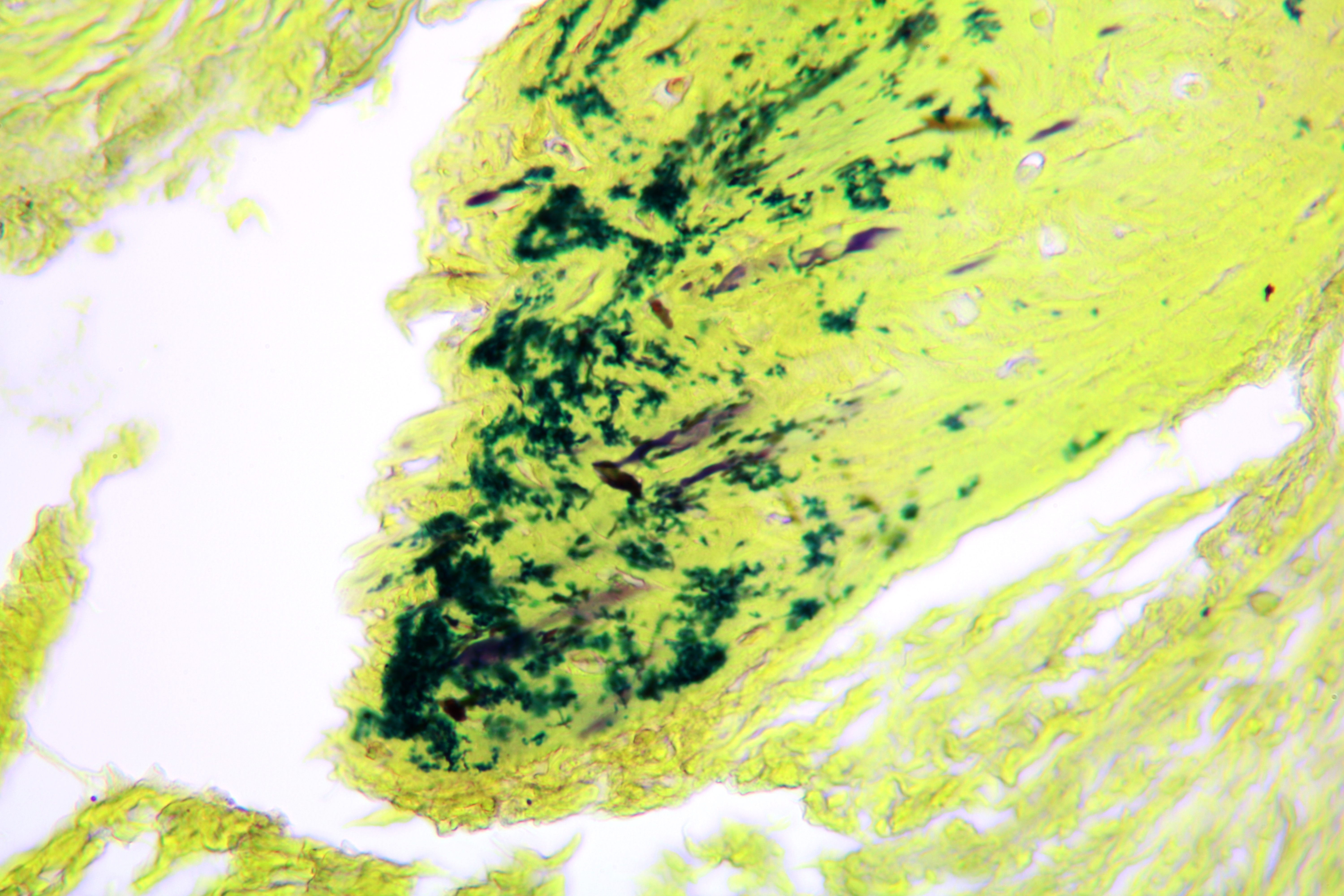
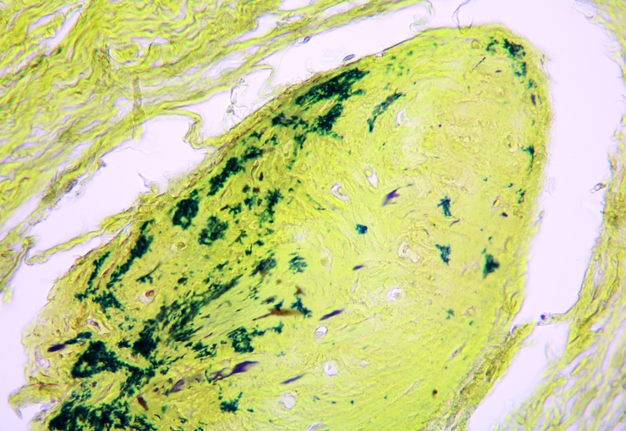
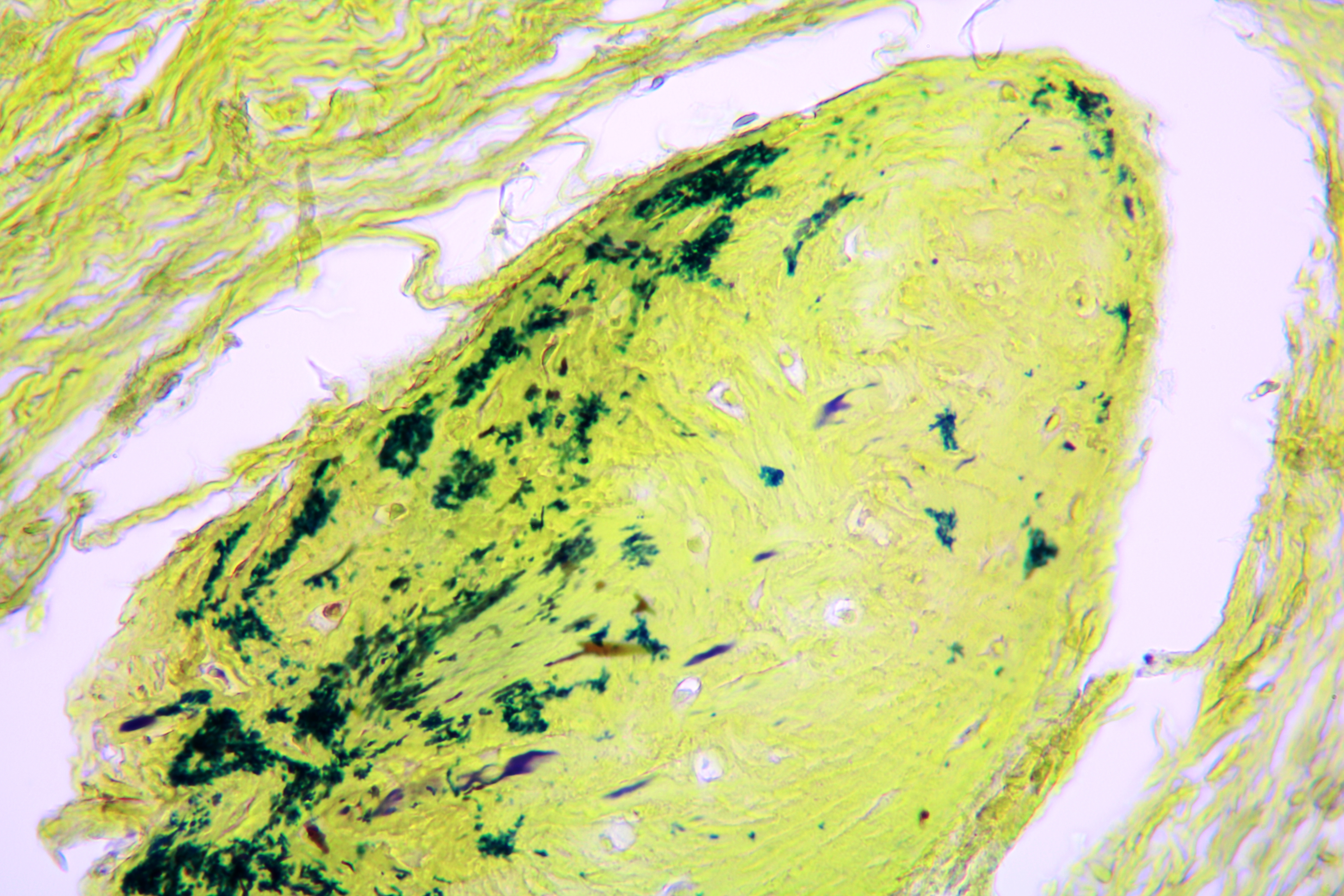
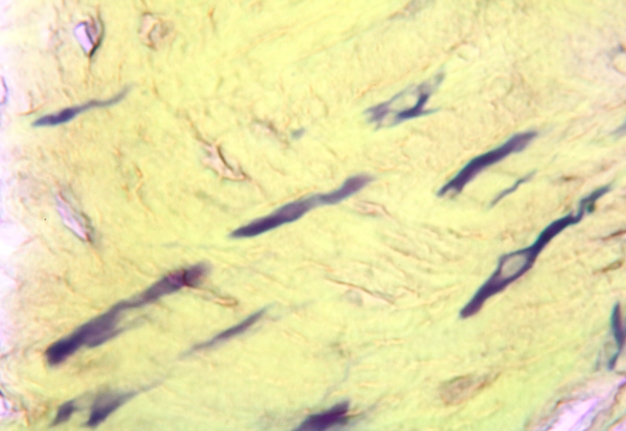
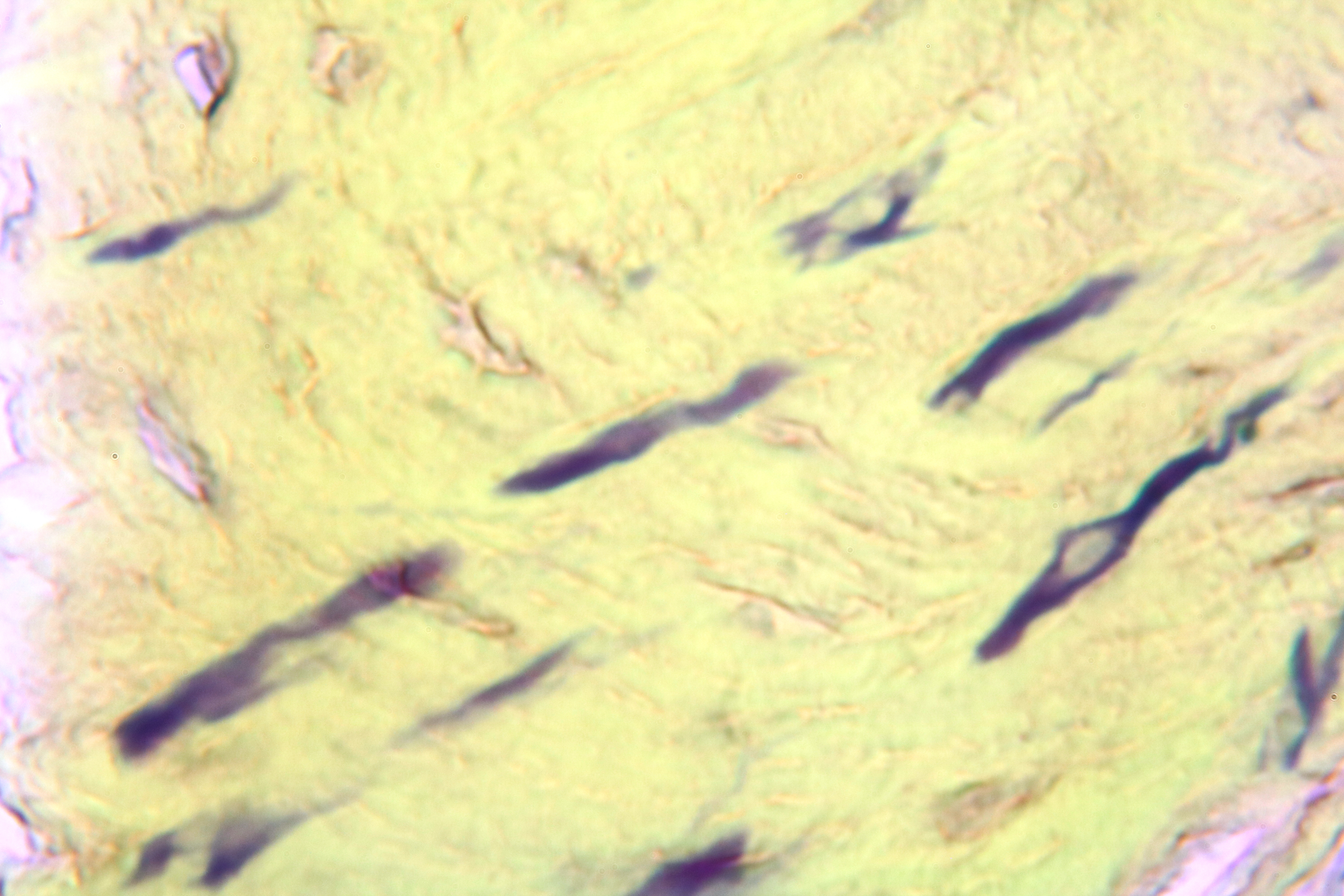
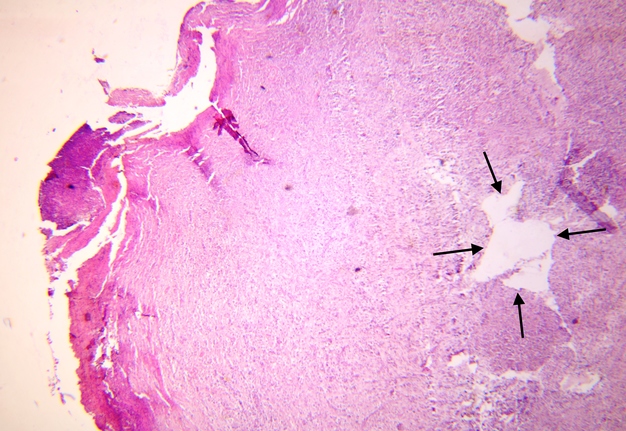
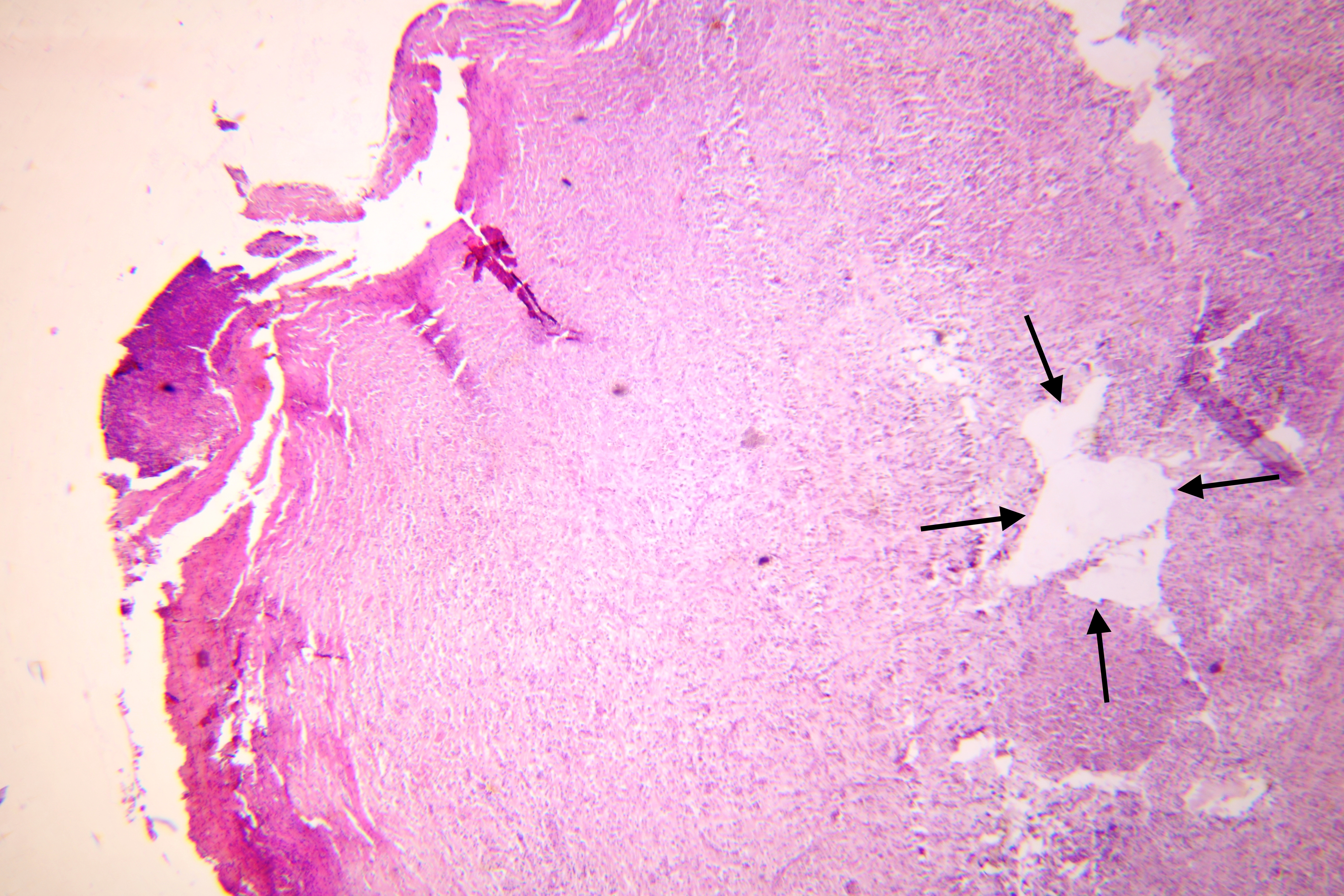
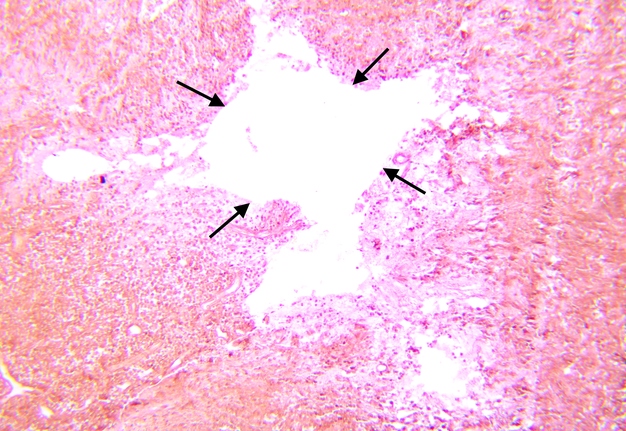
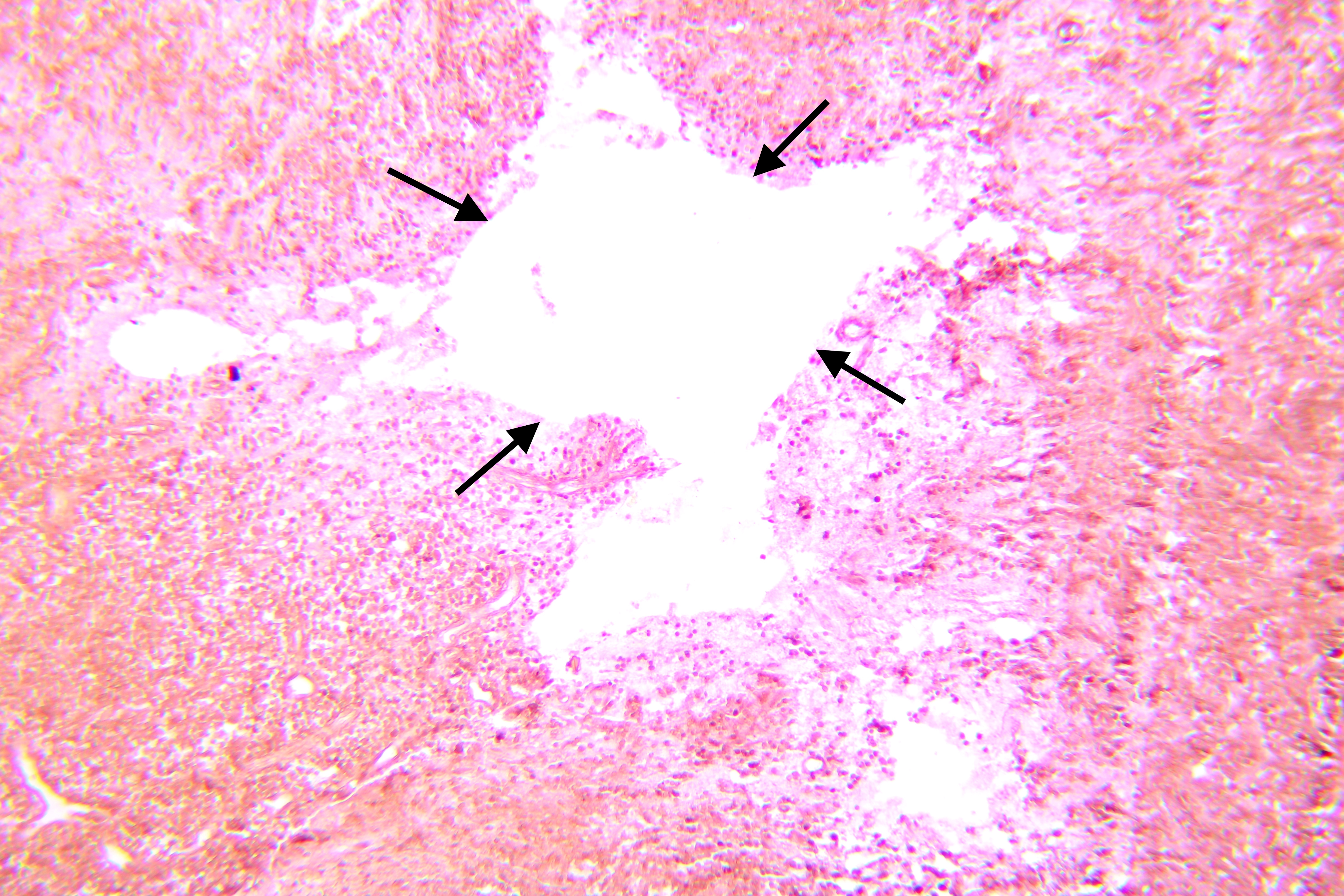
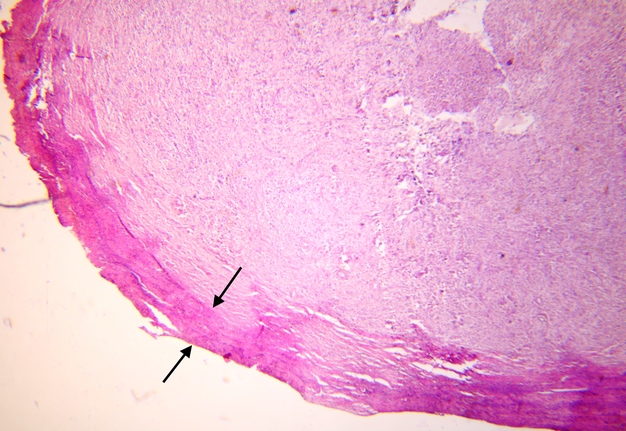
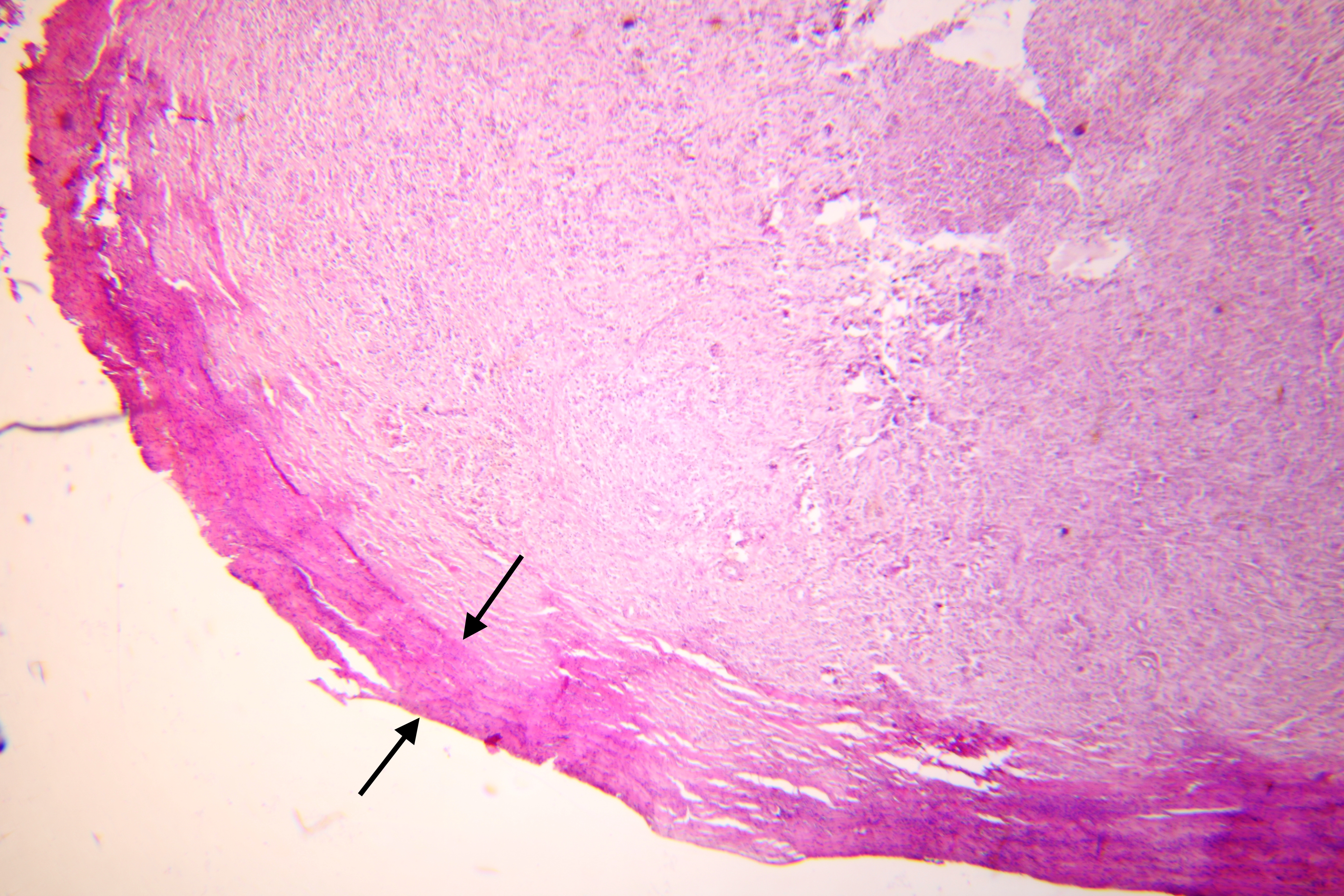
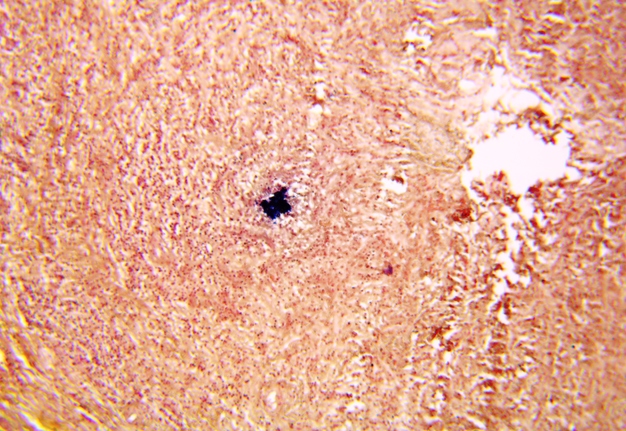
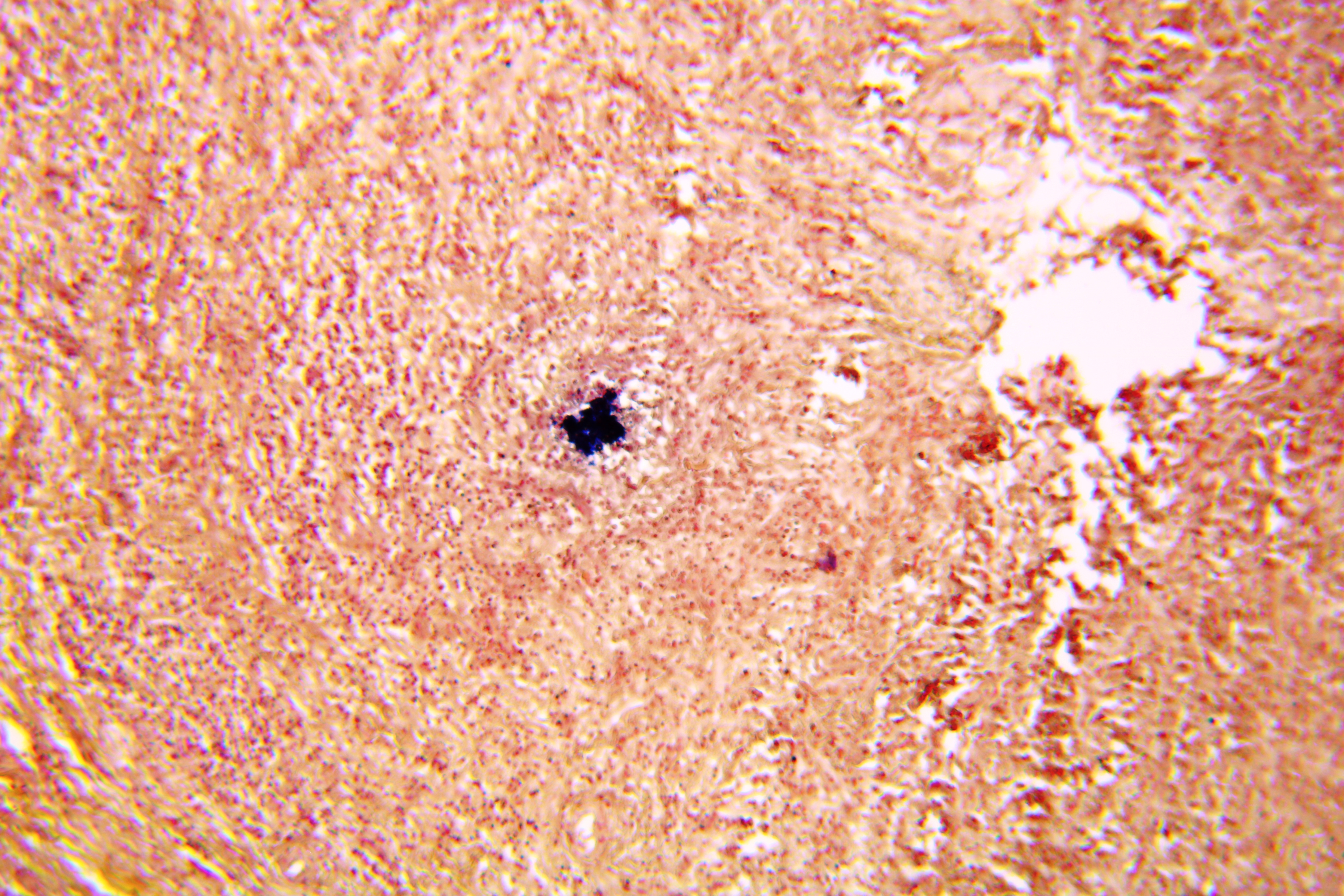
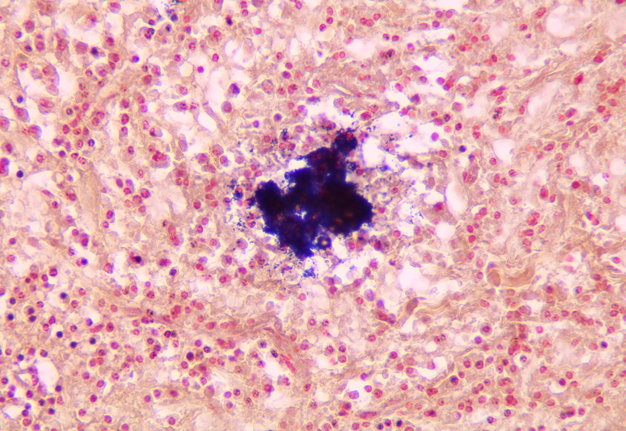
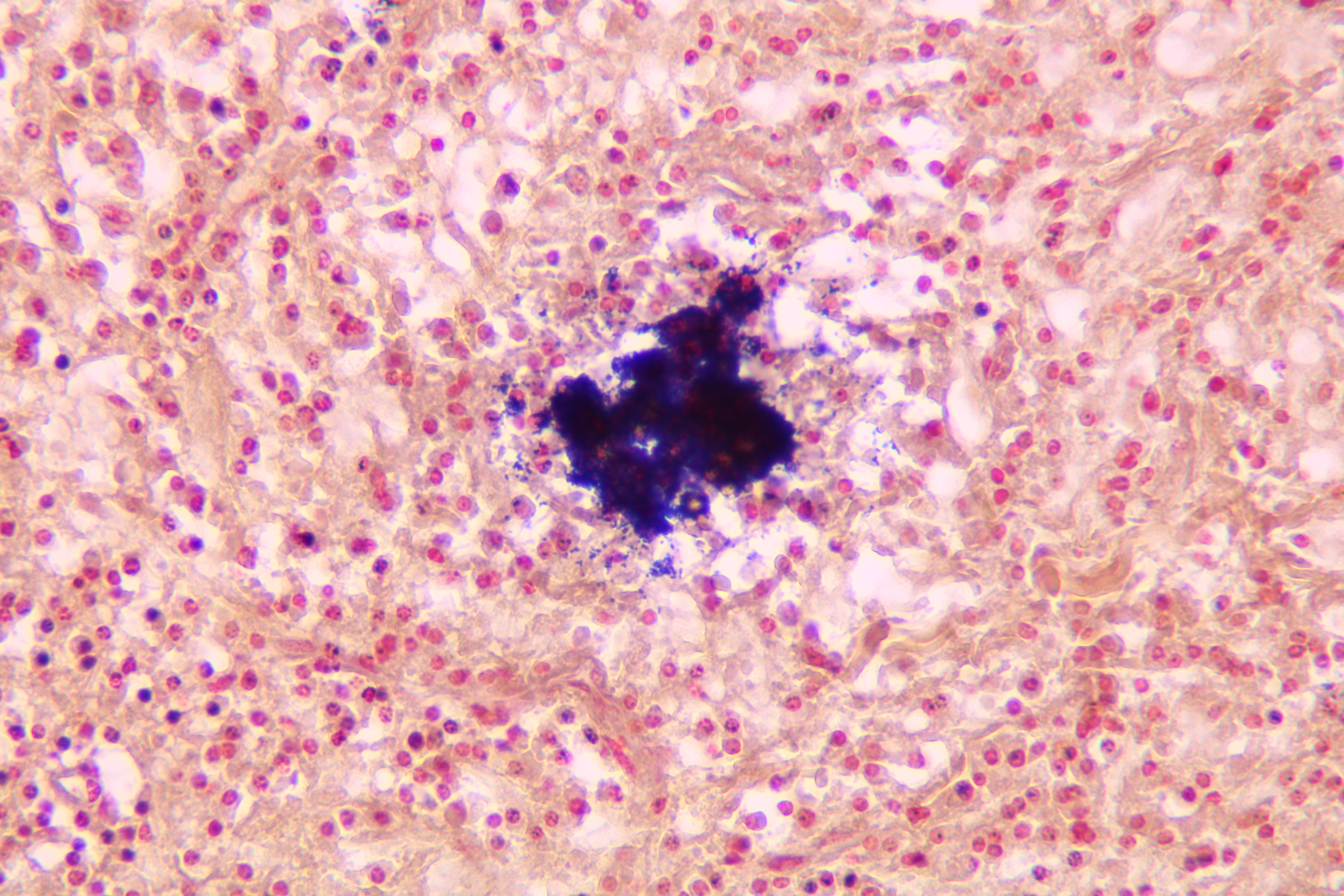
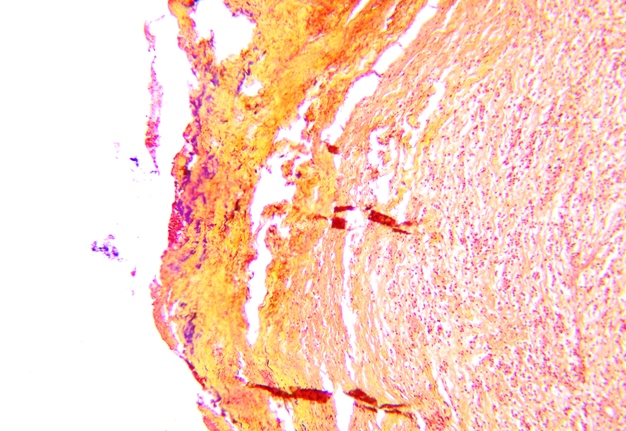
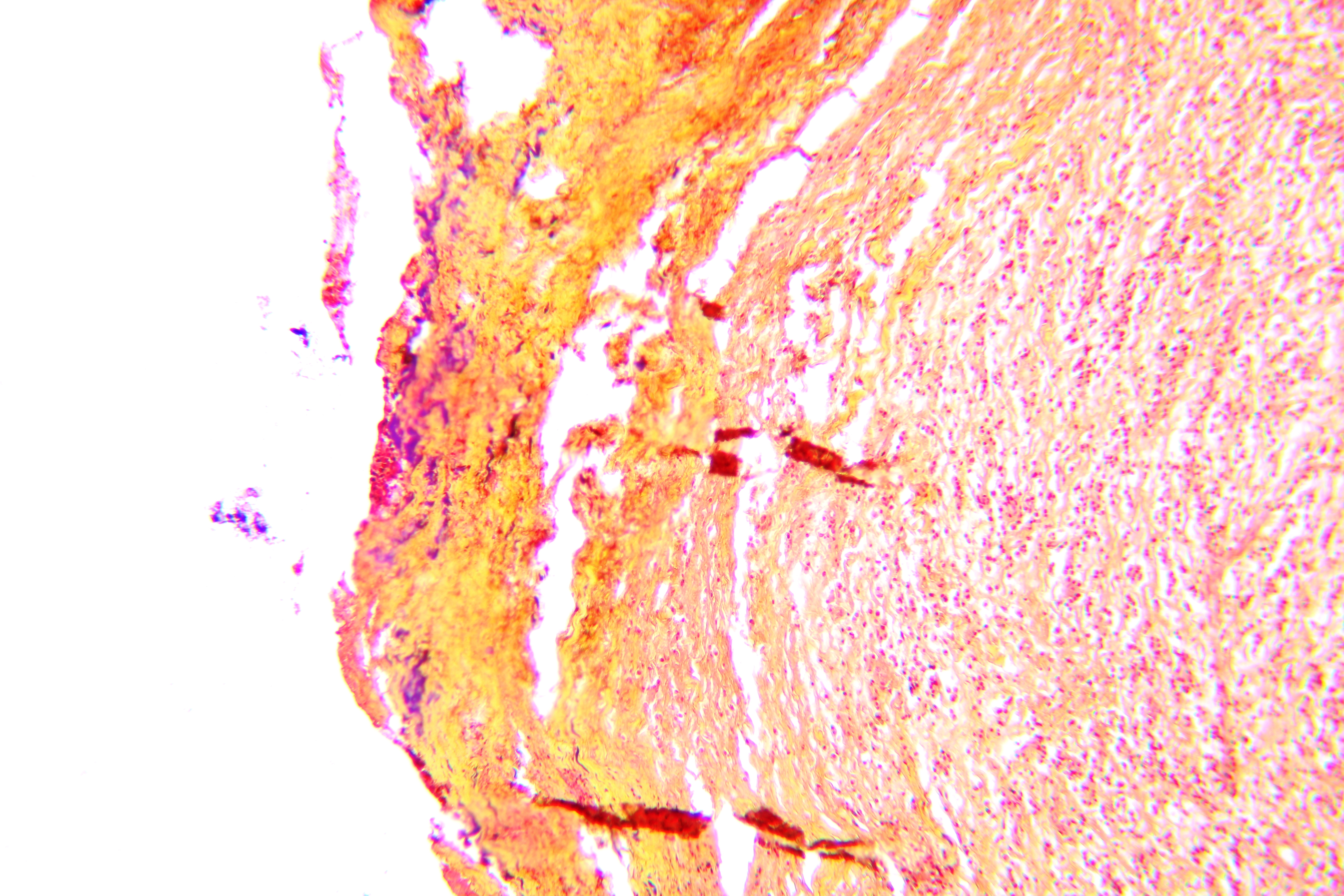
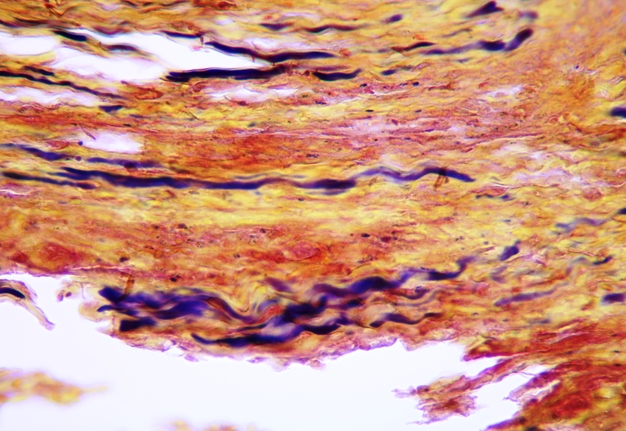
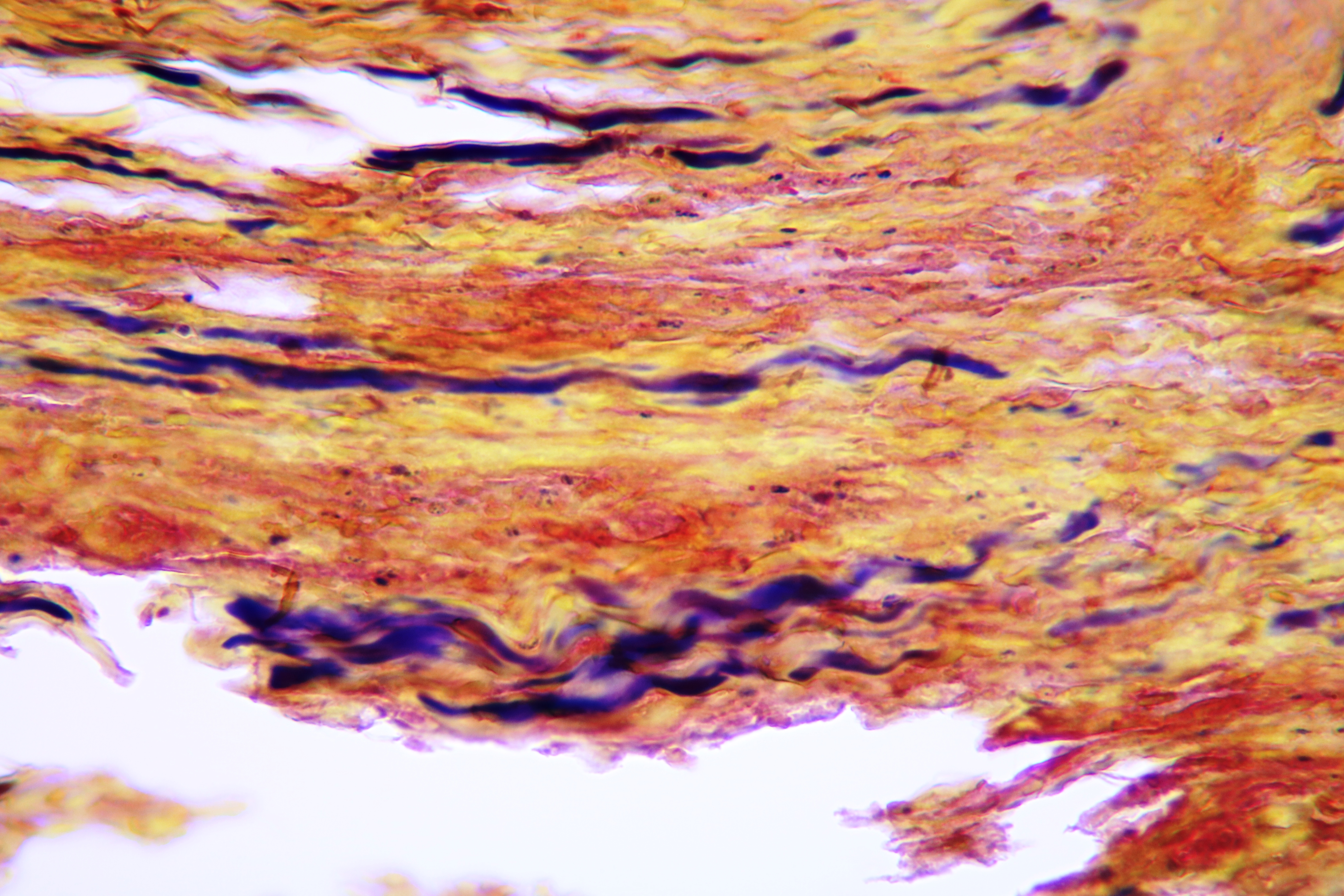
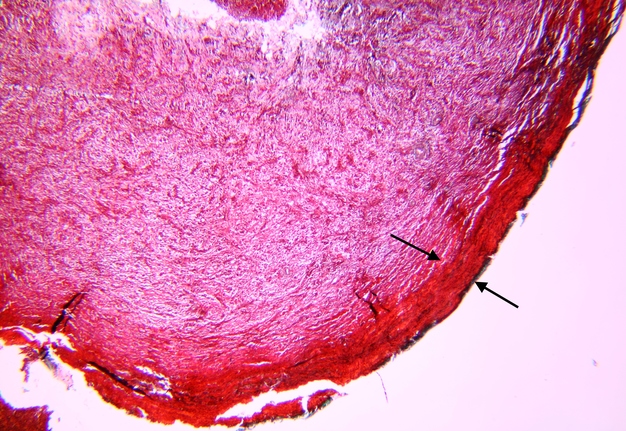
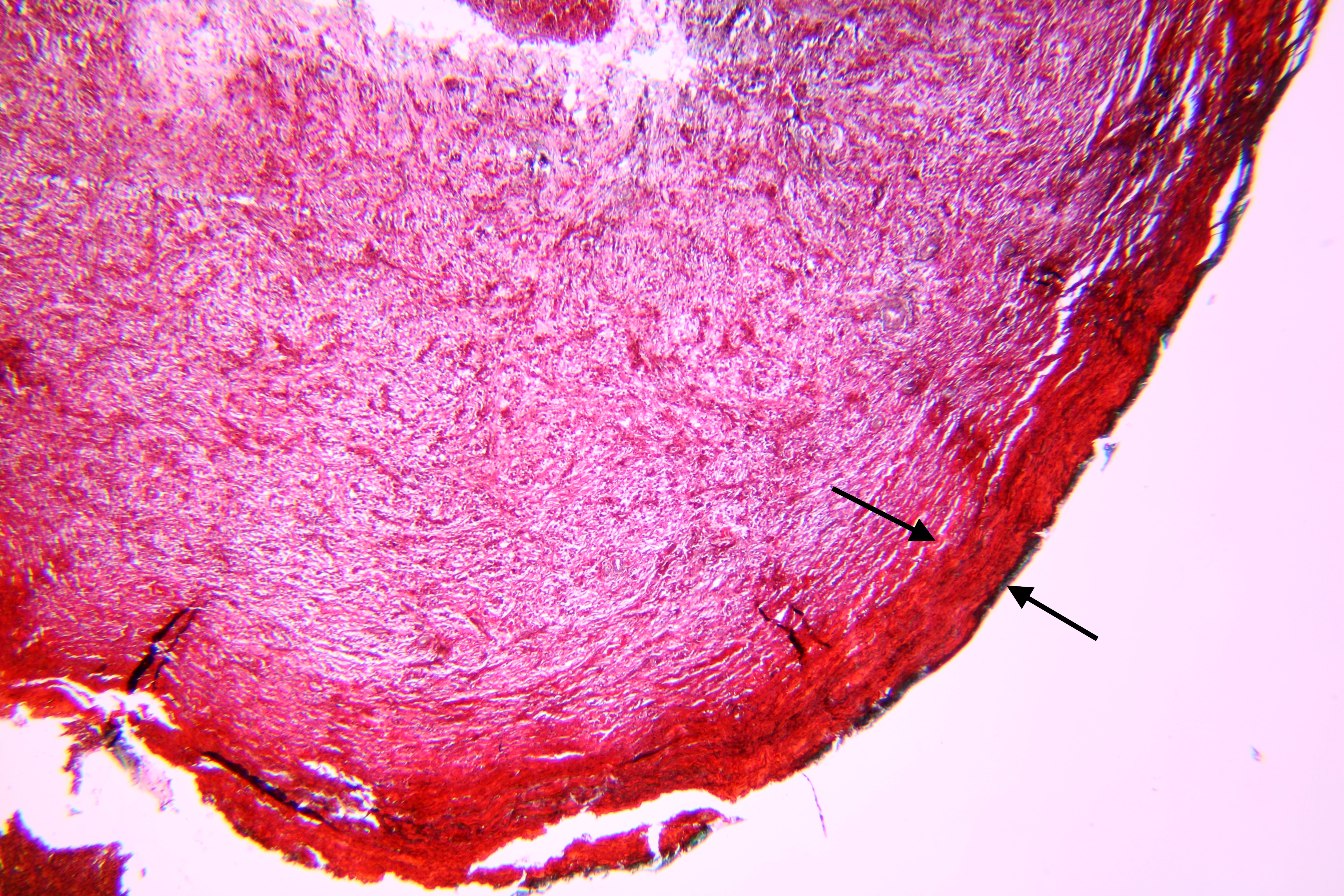
Pathohistological diagnoses were established by A.V. (his experience in periapical pathohistology is 13 years) using Carl Zeiss Primo Star laboratory microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) and confirmed at the department of pathology. Periapical scar consisting of dense fibrous connective tissue and an area of osteomyelitis have been established as pathohistological diagnosis in the area of the bone defect between the teeth 12 and 11. Dense fibrous connective tissue and bone microsequestration were visualized in the specimen. Figures 11-19 demonstrate pathohistological findings using different staining (hematoxylin and eosin, Masson’s trichrome stain in Goldner’s modification with light green, and Brown-Brain staining) and magnification (50x, 100x, and 200x).
Non-epithelialized granuloma with abscessation was established as a pathohistological diagnosis in the bone defect area near the tooth 22 apex. The granulation tissue containing an area of necrosis was noted in the specimen. The Figures 20-27 show histological findings of the specimen with hematoxylin-eosin staining, Brown-Bren staining, and Masson’s trichrome stain in Goldner’s modification with light green.
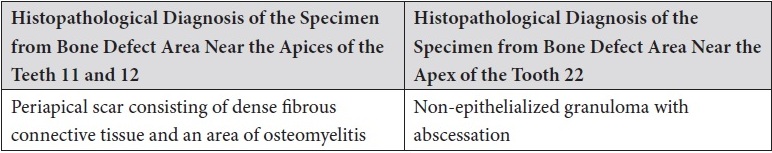
The comparison of histopathological results is highlighted in the Table 2.
TABLE 2. Comparison of Histopathological Results in the Area of Teeth 11, 12 and Tooth 22.
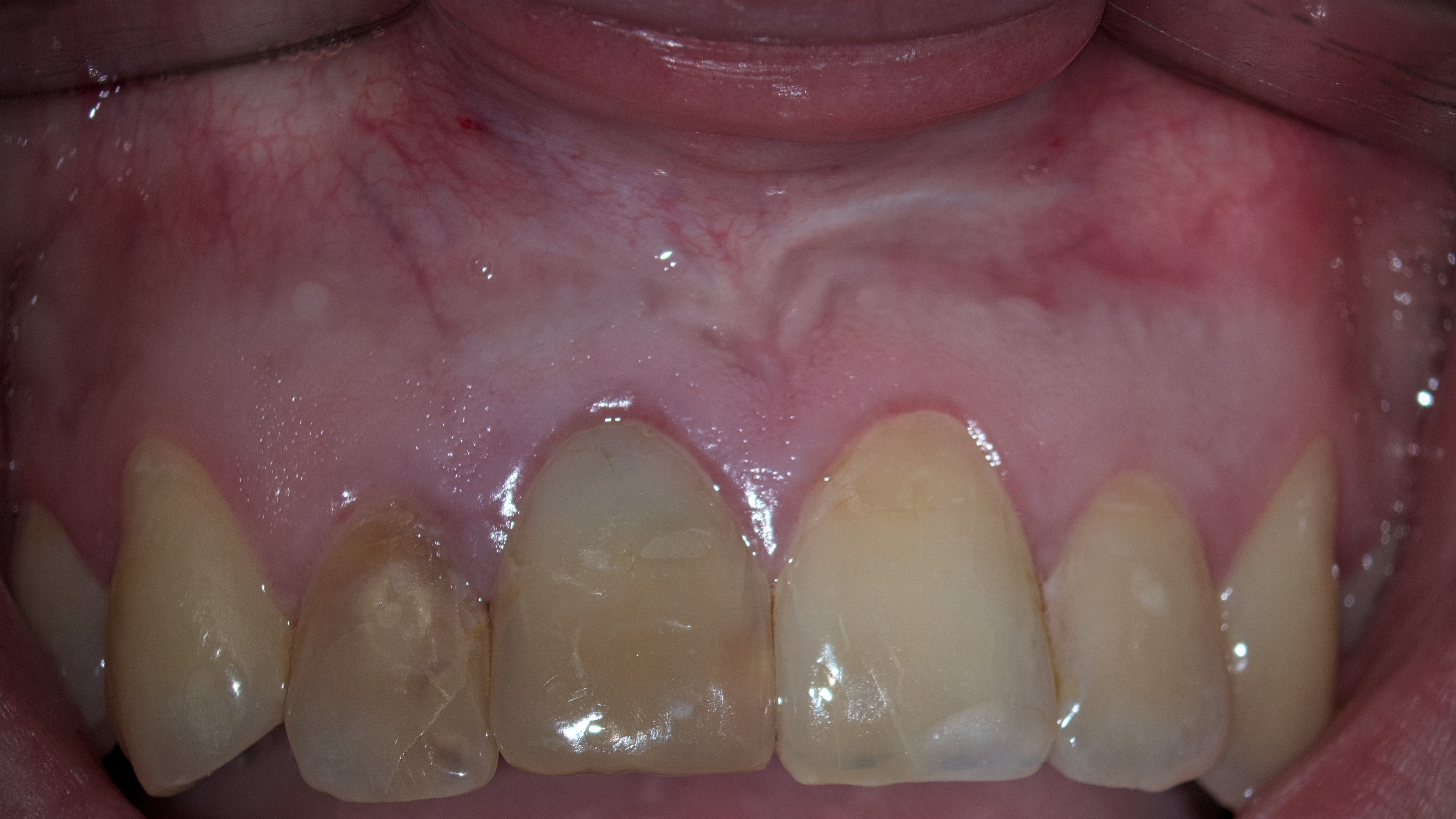
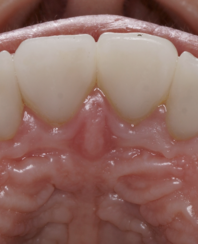
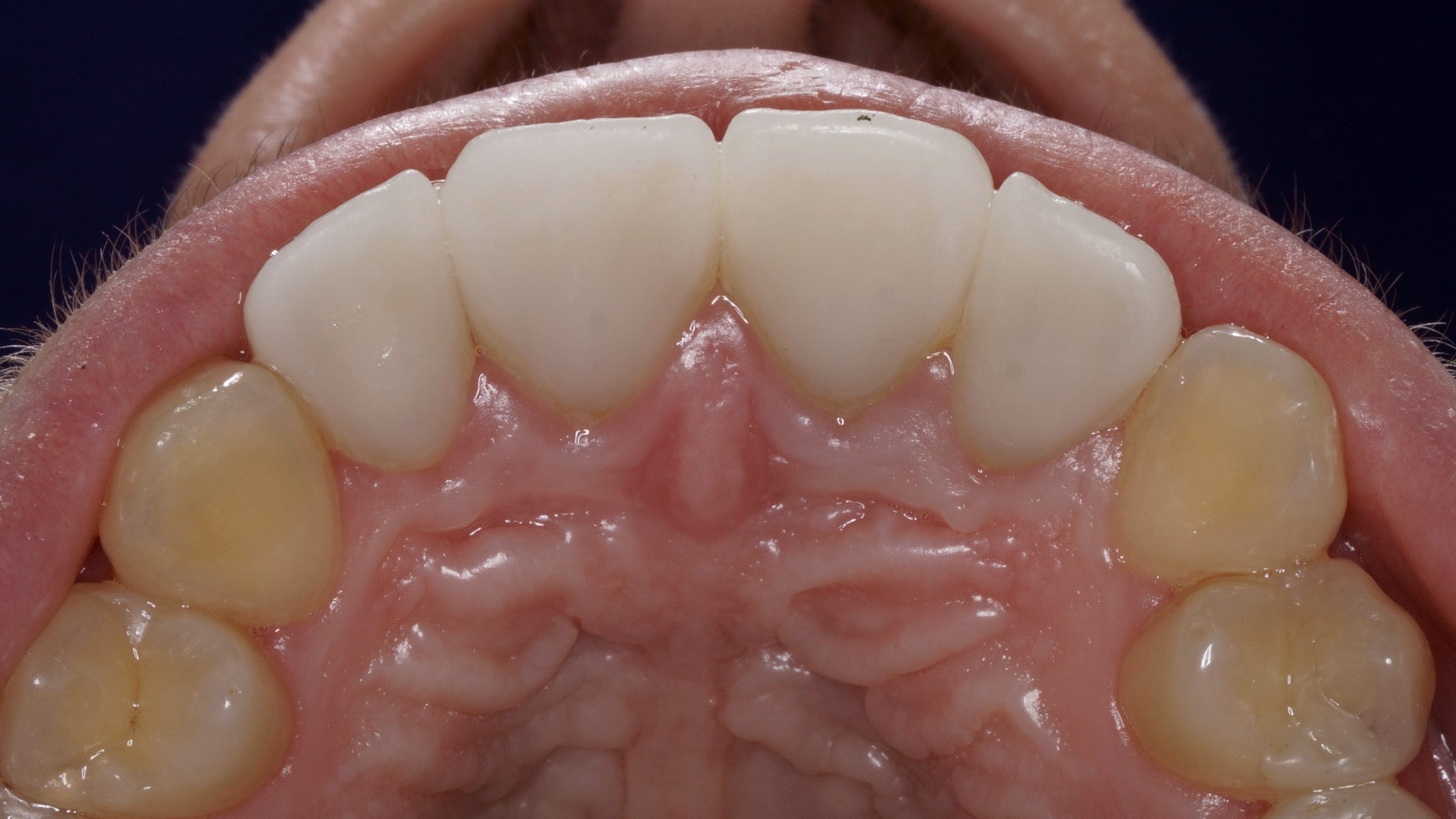
A 1.5-year post-microsurgery examination and survey (Fig 28) showed the absence of symptoms, normal appearance of the gingiva from the vestibular and palatal aspects. Temporary crowns, which are visualized on teeth 12, 11, 21, and 22, will be replaced by permanent crowns in the near future.
X-ray (Fig 29) and CBCT (Fig 30) after 1 year and 6 months show signs of incomplete healing (according to Rud and colleagues (1972) [20] and Molven and colleagues (1987) [21] classification) in the area of teeth 11, 12, and 22. It is worth noting that incomplete healing in endodontic microsurgery is evaluated as success [22].
FIGURE 29. X-ray (A, B) and CBCT (Fig 30B) after 1 year and 6 months show signs of incomplete healing (according to Rud and colleagues (1972) [20] and Molven and colleagues (1987) [21] classification) in the area of teeth 11, 12, and 22. It is worth noting that incomplete healing in endodontic microsurgery is evaluated as success [22]. 12, upper right lateral incisor; 11, upper right central incisor; 22, upper left lateral incisor.
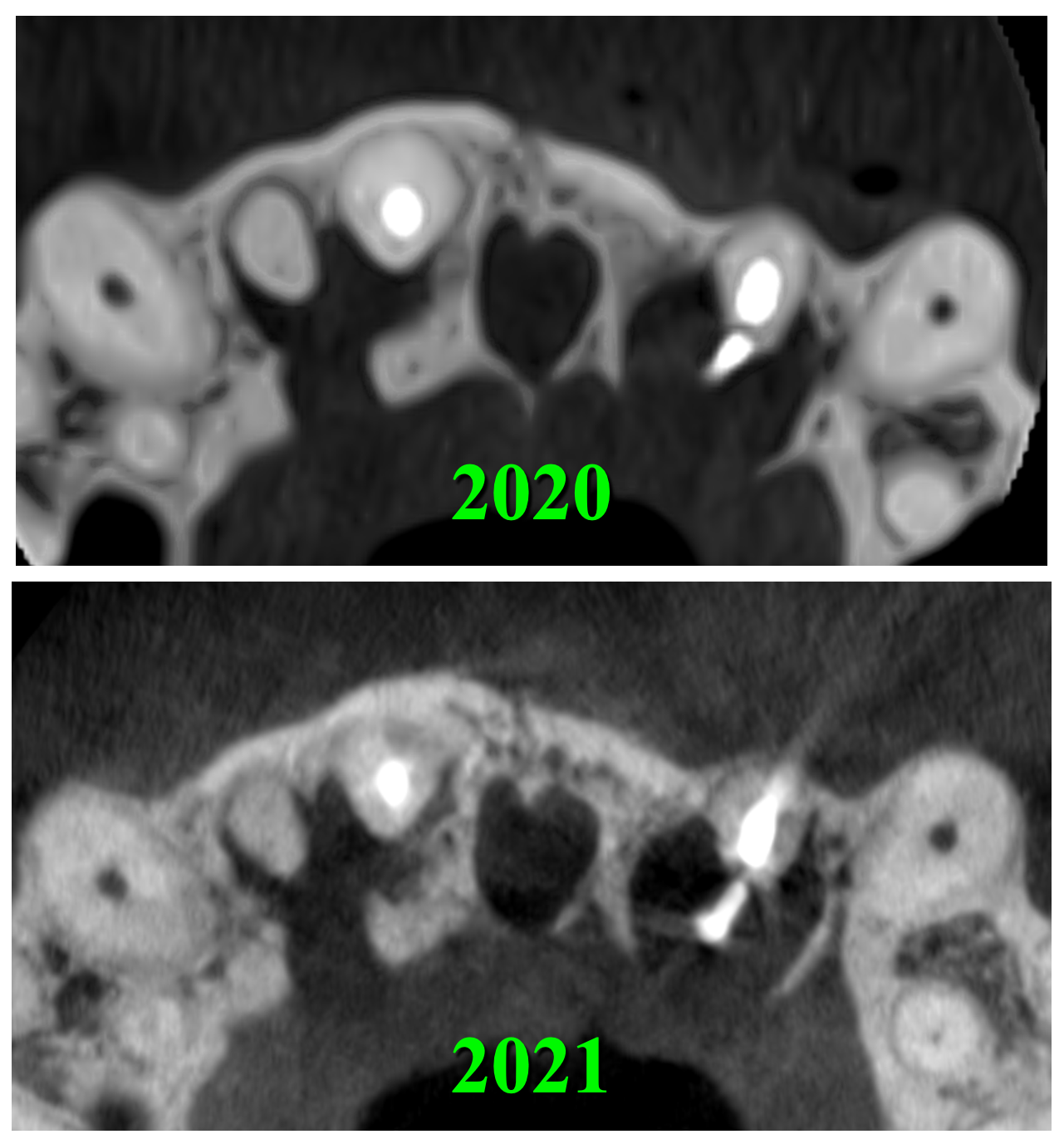
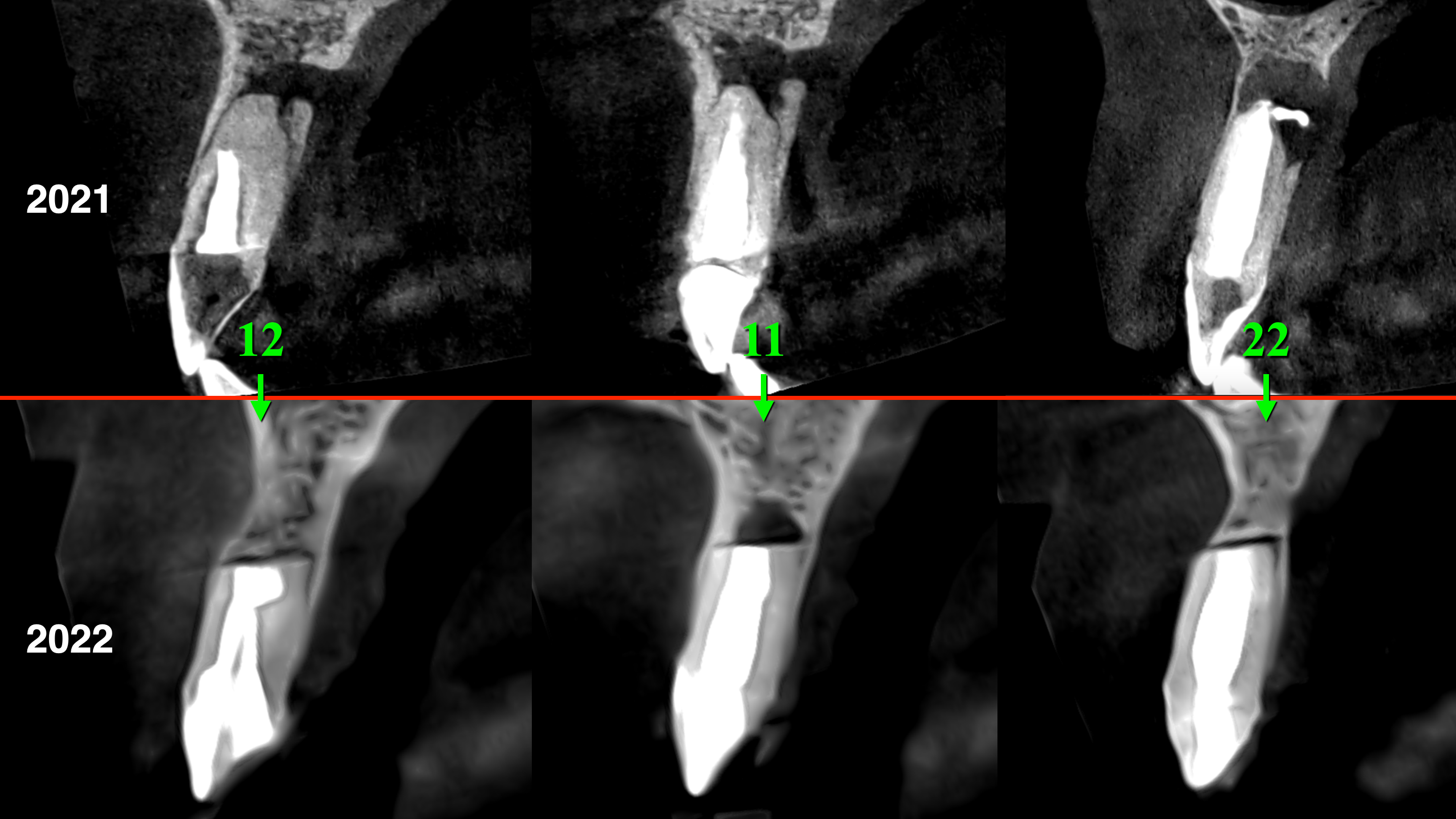
Comparing sagittal CBCT scans before (Fig 30A) and 1.5 year after (Fig 30B) the endodontic microsurgery noted the post-operative repair of the vestibular and palatal cortical plates along with partial repair of cancellous bone substance.
FIGURE 30. Sagittal CBCT scans before (A: 2021) and 1.5 year after (B: 2022) the endodontic microsurgery. Notes a repair of the vestibular and palatal cortical plates along with partial repair of cancellous bone substance. Repaired bone areas are indicated by arrows. 12, upper right lateral incisor; 11, upper right central incisor; 22, upper left lateral incisor.
Guided by the modern “3D criteria for the success of healing after endodontic microsurgery of the University of Pennsylvania” based on the CBCT obtained after 1 year and 6 months, it can be stated that the existing type of healing belongs to the category of limited healing [19].
DISCUSSION
This case illustrates the value and importance of history taking, careful clinical and radiographic evaluation for accurate diagnosis and appropriate management.
An endodontically treated tooth that has a periapical lesion in the form of radiolucency that exists for a long time and does not heal, but at the same time does not bother the patient, for some reason is often interpreted by doctors as a periapical pathology and, accordingly, wrong decisions are made regarding treatment. The small amount of information about the apical/periapical scar in endodontically treated teeth with its characteristic clinical and radiographic features causes the difficulty of differential diagnosis between the apical/periapical scar and various apical/periapical lesions that look like periapical radiolucency. Many scientific sources do not consider an apical/periapical scar to be a pathology that requires treatment [7]. Moreover, it is generally accepted that scar healing is a success in endodontic microsurgery [22]. The listed general clinical and radiological signs of apical/periapical scars according to the data of Lee and colleagues [7] make possible to diagnose apical/periapical scars more accurately and make decisions about the expediency of treatment.
CONCLUSIONS
The purpose of this clinical case report was to provide education and awareness regarding apical scarring occurring in traumatized teeth, subsequent endodontic treatment, and endodontic microsurgery. The importance of careful clinical and radiographic evaluation and biopsy submission for accurate diagnosis and treatment was emphasized.
AUTHOR CONTRIBUTIONS
Conceptualization: Tkachenko O, Volokitin A. Data acquisition: Tkachenko O, Volokitin A. Data analysis or interpretation: Tkachenko O, Volokitin A. Drafting of the manuscript: Volokitin A, Tkachenko O. Critical revision of the manuscript: Tkachenko O, Volokitin A. Approval of the final version of the manuscript: both authors.
Open Researcher and Contributor Identity Document (ORCID)
-
Oleksandr Tkachenko, https://orcid.org/0000-0003-2582-4551
-
Alexey Volokitin, https://orcid.org/0000-0001-5977-8608
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES (22)
-
Penick EC. Periapical repair by dense fibrous connective tissue following conservative endodontic therapy. Oral Surg Oral Med Oral Pathol. 1961;14(2):239-242. Crossref
-
Seltzer S, Bender IB, Smith J, Freedman I, Nazimov H. Endodontic failures--an analysis based on clinical, roentgenographic, and histologic findings. I. Oral Surg Oral Med Oral Pathol. 1967;23(4):500-516. Crossref
-
Nair PN, Sjögren U, Figdor D, Sundqvist G. Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(5):617-627. Crossref
-
Bhaskar SN. Periapical lesion: types, incidence, and clinical features. Oral Surg Oral Med Oral Pathol. 1966;21(5):657-671. Crossref
-
Nair PNR. On the causes of persistent apical periodontitis: a review. Int Endod J. 2006;39(4):249-281. Crossref
-
American Association of Endodontists (AAE). Glossary of endodontic terms. 10th edition. Chicago: AAE; 2020.
-
Lee YP, Hwang MJ, Wu YC, Lang MJ, Wu YH, Chiang CP. Clinicopathological study of periapical scars. J Dent Sci. 2021;16(4):1140-1145. Crossref
-
Neville B, Damm DD, Allen CM, Chi AC. Oral and maxillofacial pathology. 4th ed. St. Louis: Elsevier; 2015.
-
Stockdale CR, Chandler NP. The nature of the periapical lesion--a review of 1108 cases. J Dent. 1988;16(3):123-129. Crossref
-
Spatafore CM, Griffin JA, Keyes GG, Wearden S, Skidmore AE. Periapical biopsy report: an analysis over a 10-year period. J Endod. 1990;16(5):239-241. Crossref
-
Nobuhara WK, Del Rio CE. Incidence of periradicular pathoses in endodontic treatment failures. J Endod. 1993;19(6):315-318. Crossref
-
Liapatas S, Nakou M, Rontogianni D. Inflammatory infiltrate of chronic periradicular lesions: an immunohistochemical study. Int Endod J. 2003;36(7):464-471. Crossref
-
Becconsall-Ryan K, Tong D, Love RM. Radiolucent inflammatory jaw lesions: a twenty-year analysis. Int Endod J. 2010;43(10):859-865. Crossref
-
Peñarrocha M, Carrillo C, Peñarrocha M, Peñarrocha D, von Arx T, Vera F. Symptoms before periapical surgery related to histologic diagnosis and postoperative healing at 12 months for 178 periapical lesions. J Oral Maxillofac Surg. 2011;69(6):e31-e37. Crossref
-
Çalışkan MK, Kaval ME, Tekin U, Ünal T. Radiographic and histological evaluation of persistent periapical lesions associated with endodontic failures after apical microsurgery. Int Endod J. 2016;49(11):1011-1019. Crossref
-
García-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112-121. Crossref
-
Liu H, Li D, Zhang Y, Li M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem Cell Biol. 2018;149(4):393-404. Crossref
-
Horka E, Foltanb R, Hanzelka T, Pavlikova G, Klima K, Sedy J. Etiopathogenesis of post-endodontic periapical scar formation. Dent Hypotheses. 2012;3(1):5-16.
-
Kim S, Kratchman S. Microsurgery in endodontics. 1st ed. Hoboken: Wiley; 2018. Crossref
-
Rud J, Andreasen JO, Jensen JE. Radiographic criteria for the assessment of healing after endodontic surgery. Int J Oral Surg. 1972;1(4):195-214. Crossref
-
Molven O, Halse A, Grung B. Observer strategy and the radiographic classification of healing after endodontic surgery. Int J Oral Maxillofac Surg. 1987;16(4):432-439. Crossref
-
Tsesis I. Complications in endodontic surgery. Berlin Heidelberg: Springer-Verlag; 2014. Crossref